Synchronous Primary Endometroid Ovarian and Endometrial Carcinomas - Pathohistological Criteria for Differential Diagnosis, Clinical Characteristic and Treatment Behavior
Lena Marinova*, Vaska Vasileva and Iliya Gabrovski
Medical Oncology Clinic, Department of Radiation Oncology and Metabolic Brachytherapy, UMHAT “Queen Joanna” Sofia, Bulgaria
Received Date: 05/05/2023; Published Date: 17/08/2023
*Corresponding author: Lena Marinova, Medical Oncology Clinic, Department of Radiation Oncology and Metabolic Brachytherapy, UMHAT “Queen Joanna” Sofia, Bulgaria
Abstract
Synchronous primary carcinomas of the female genital tract are rare comprising only about 1% of all genital malignancies. We present a 64 -year menopausal patient with synchronous primary endometrial and ovarian carcinoma. An operation was performed, including the classic total laparohisterectomy without a lymphatic pelvic dissection. The main diagnostic problem is the differential diagnosis between synchronous tumor primacy in the ovary and endometrium from a local advanced endometrial tumor with hematogenous metastases in the ovary.
Macroscopic pathohistological and immunohistochemical criteria are significant factors for determining the prognosis and strict definition of the clinical stage and the need for adjuvant postoperative treatment.
Keywords: Synchronous endometroid carcinomas; Endometroid endometrial carcinoma; Endometroid ovarian carcinoma; Immunohistochemical analysis; Surgery; Radiotherapy
Introduction
Synchronous tumors of the female genital tract are rare comprising only about 1% of all genital malignancies [1-3]. Their pathogenesis is not clear. Tumor synchrony is most likely due to a combination of embryonic, hormonal and genetic factors [4,5]. As the incidence of synchronous primary endometrial and ovarian carcinoma (SPEOC) is limited, it can easily be confused with endometrial cancer with ovarian metastasis [6]. The main diagnostic problem is the differential diagnosis between synchronous tumor primacy in the ovary and endometrium from a local advanced endometrial tumor with hematogenous metastases in the ovary.
Clinical Case
We present a 64 -year menopausal patient with complaints of genital bleeding and pelvic pain. An operation was performed, including the classic total laparohisterectomy without a lymphatic pelvic dissection. Two endometroid tumors are diagnosed pathologically- one in the endometrium and one in the ovary. Histological result: uterine body - moderately differentiated endometroid carcinoma of the endometrium with infiltration of 2/3 of the myometrium (Figure 1); adenomatous polyp of the cervical canal; in the area of the cervix -epithelial glandular -papillary erosion. Immunohistochemical (IHC) analysis: Estrogen receptor (ER)-positiv; Vimentin- positiv (Figure 2) /рT1в G2. Left ovary- moderately differentiated endometroid ovarian adenocarcinoma without infiltration in the ovarial capsule (Figure 3). The tumor infiltration does not reach the outer layer of the cyst wall. Right ovary- cortex hyperplasia, inclusion and chocolate cyst, ovarian endometriosis. Immunohistochemical (IHC) analysis: Estrogen receptor (ER)-negativ (Figure 4)/ pT1a G2. The patient was presented to the Oncology Committee and judged as adjuvant complex treatment, including chemotherapy (Ch) and postoperative radiotherapy (RT). 3 courses Ch were carried out, followed by Intensity Modulated Radiotherapy (IMRT) with VMAT technique in the tumor bed with the above 2/3 of the vagina and the pelvic lymph nodes with Daily Dose (DD) 2 Gy up to total dose (TD) 50 Gy and 3 courses adjuvant Ch (Figure 5). After completion of the complex treatment, the patient was referred for control gynecological examinations.
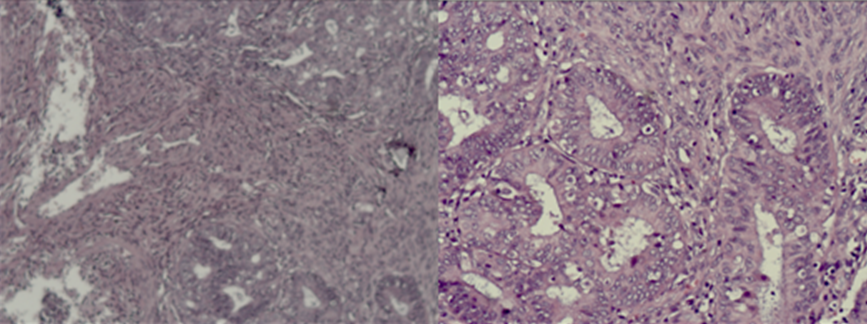
Figure 1: Photomicrography- Moderately differentiated endometroid adenocarcinoma of the endometrium with infiltration of 2/3 of the myometrium/ H&E 10 х; H&E 20 х.
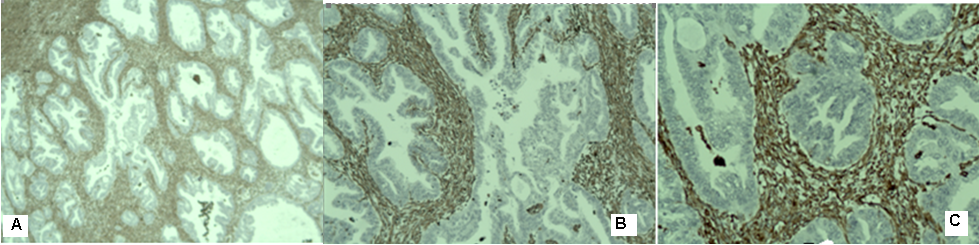
Figure 2: Photomicrography – Immunohistochemical analysis of endometrioid endometrial adenocarcinoma - А/ ЕR positive expression х 10.; Б/ Vimentin positive expression х10; В/ Vimentin positive expression х 20.

Figure 3: Photomicrography of a left ovary - Moderately differentiated endometroid ovarian adenocarcinoma without infiltration in the ovarial capsule А/H&E 10 х; В/ H&E 20 х.
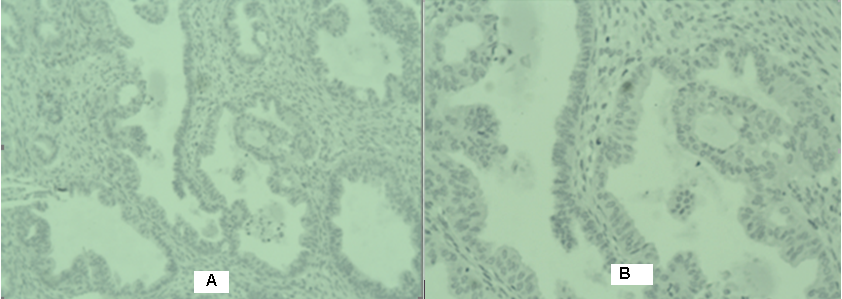
Figure 4: Photomicrography - Immunohistochemical analysis of moderately differentiated endometroid ovarian adenocarcinoma- A/ ЕR negative expression х10; В/ ЕR negative expression х20.

Figure 5: Intensity modulated radiotherapy with VMAT technique in the tumor bed with the above 2/3 of the vagina and the pelvic lymph nodes with DD 2 Gy up to TD 50 Gy.
Discussion
The endometroid subtype of the primary tumors is the most common histological finding which is found in 50%–70% of cases and the primary independent tumors are often grade 1 or 2 [7]. Among them, synchronous endometrial and ovarian tumors are the most common types of malignancy, with a frequency of 5% among endometrial and 10% among ovarian primary tumors [8,9]. Synchronous endometroid adenocarcinomas are classified into three groups: 1/ Synchronous primary tumors (Figure1, 3); 2/ Primary endometrial carcinoma with metastasis in the ovary; 3/ Primary ovarian carcinoma with metastasis in the endometrium [10]. In primary endometrial carcinoma with metastasis in the ovary, several pathohistological characteristics are important:1/ large volume of endometrial tumor with a small ovarian tumor; 2/ additional presence of atypical endometrial hyperplasia; 3/ deep myometral tumor invasion, accompanied by vascular invasion and local distribution in the adnex; 4/ bilateral ovarian tumors and/ or multinodular; 5/ lack of ovarian endometriosis [11]. In primary ovarian carcinoma with metastasis in the endometrium, several pathohistological characteristics are typical: 1/ large ovarian tumor - small endometrial tumor; 2/ presence of ovarian endometriosis; 3/ unilateral ovarian tumor in 80-90% of cases; 4/ lack of atypical hyperplasia in the endometrium [11]. In the primary endometrial and primary ovarian carcinomas, such pathohistological tumor characteristics aneuploidy / heteroploidy with similar DNA changes or diploids of both tumors and similar molecular -genetic or karyotype changes are detected. Age is a significant factor for high levels of hormonal ER receptors (P = 0.007) in endometrial carcinomas. The relapses and the lethal outcome correlate significantly with low levels of ER and PR (p <0.01) [12]. IHC analysis of ER and PR of endometroid endometrial and ovarian tumors distinguishes the primary tumor synchronicity from distant metastasis. In metastatic endometrial and ovarian carcinomas, the same ER and PR receptors levels are detected, and in synchronous primary tumors they are different [13,14]. 62.5% of the simultaneous endometrial and ovaryal neoplasms are significantly distinguished from the metastatic by different immunohistochemical expression of ER and PR receptors (P = 0.0006), and in 31.3% of cases a Differential Diagnosis (DD) is required via BCL-2 (P = 0.03). The DD is not applicable to the IHC of Her-2 /Neu, P53 and KI-67 [14]. In our clinical case, a different hormonal estrogen and progesterone IHC expression is demonstrated, which proves the synchronous tumor primacy (Figure 2, 4). In synchronous endometroid tumor primacy, a number of most common clinical and pathochistological characteristics are reported in the endometrium and ovarium: 1/ Endometrial tumor- G1 endometroid tumors in 57%, without vascular invasion in 85.8%, with myometral wall invasion under ½ in 71.4%, lack of endometrial hyperplasia in 57% [15]. 2/ Ovarian tumor- endometroid tumors, unilateral ovarian tumor in 80-90% of cases, there is no tumor infiltration in the ovarian surface epithelium; without invasion of the fallopian tube; without vascular invasion of 71.4%. The opinion of the different authors differs about the lack of ovarian endometriosis [11,15]. In the clinical case we have presented, the patient is in postmenopausal age, which is associated with the presence of ovarian endometriosis. As with the clinical case presented, in many authors, synchronous primary carcinomas are mainly in an early clinical stage [16-18]. Published literature has reported better outcomes in the synchronous disease category as compared to the groups with a single primary having metastatic disease [19]. Endometrioid histology has been shown to have a better prognosis than non-endometrioid histology [10,20]. In synchronous tumor primacy, systematic surgical staging is the mainstay of the management for such patients and often includes total abdominal hysterectomy with bilateral salpingo‐oophorectomy, total omentectomy, appendectomy, pelvic and para‐aortic lymphadenectomy, and complete resection of all diseases with biopsy of suspect lesions and pelvic lavage [21-27]. It is well known that optimal cytoreductive surgery (resected to <1 cm gross residual disease) in ovarian cancer is associated with improved survival [28] and, while the data are more limited in endometrial cancer, these findings are often extrapolated from ovarian cancer [21,29]. The main question in the healing tactics is the need for adjuvant postoperative treatment /Radiotherapy (RT) and /or Chemotherapy (Ch) in both early endometroid neoplasms (clinical stage IB and IA G1-2)? However, no guidelines for adjuvant therapy in patients with synchronous cancers have been established yet and the treatment of respective cancer guides the adjuvant treatment. In ovarian cancer, all but stage IA/B are to receive Ch and in endometrial cancer, it is indicated when the risk of distant metastasis is high [30]. The need for adjuvant therapy for an early-stage endometrial cancer is dependent on uterine factors such as depth of invasion, lymphovascular space invasion, and histologic subtype [31]. The role of postoperative adjuvant treatment is controversial [20,21,32]. In many clinical cases, synchronous primary endometrial and ovarian tumors require an individual approach for adjuvant treatment that corresponds to the risk of recurrence of each individual carcinoma [32,33]. Meta-analysis of local and locoregional recurrences in the 1st clinical stage of endometrial carcinoma compares the data of patients with and without adjuvant RT. After adjuvant RT, a significant reduction in local recurrences is demonstrated without improved overall survival [34,35]. In order to reduce local recurrences in the lower 2/3 of the vagina, in low-risk primary endometrial carcinoma /pT1BN0/G1-2, intravaginal brachytherapy is required. The prognosis for pT1 A/B G1 ovarian carcinomas is extremely favorable after maximal surgical cytoreduction. 5 years of survival in early ovarian carcinoma I-II clinical stage reaches 70-95%. Adjuvant Ch after surgical cytoreduction in ovarian carcinoma I A/B clinical stage depends on the degree of cancer cells differentiation/G. In ovarian cancer I A/B clinical stage/G1 an adjuvant Ch is not held, and in the G2 patient is subject to observation or 3-6 courses of Ch. In our patient with two primary initial tumors (endometrial pT1B/G2 and ovarian pT1A/G2), 3 courses Ch were carried out, followed by intensity modulated radiotherapy (IMRT) with VMAT technique in the tumor bed with the above 2/3 of the vagina and the pelvic lymph nodes with daily dose (DD) 2 Gy up tu Total Dose (TD). 50 Gy (Fig.5) and 3 courses adjuvant Ch. The patient was directed for monitoring, and after 5 years after the complex treatment completion, a Local Tumor Control (LTC) with good quality of life was established. The prognosis for synchronous primary endometrial and ovarian cancers is very good. The published 5 years and 10 years of overal survival reach 85.9% and 80.3% respectively [21].
Conclusion
Synchronous primary ovarian and endometrial neoplasms are rare. They require a strict differential diagnosis, distinguishing tumor primacy from hematogenous metastatic. Macroscopic pathohistological and immunohistochemical criteria are significant factors for determining the prognosis and strict definition of the clinical stage and the need for adjuvant postoperative treatment.
References
- Sandoval Martínez D, García Ayala E, Mayorga Anaya H. Neoplasia primaria sincrónica de endometrio y ovario: a propósito de un caso. Rev Chil Obstet Ginecol, 2011; 76(2): 113‐117.
- Pasam MK, Rajendiran S, Susruthan M, Rani U. Synchronous primary ovarian mucinous carcinoma and endometrioid endometrial carcinoma: a rare case report. Ann Pathol Lab Med, 2017; 4(4): C119‐C123.
- Patru CL, Marinas MC, Drocas I, et al. Synchronous primary ovarian and endometrial carcinomas in a young PatientCase report and literature review. Rev Chim, 2020; 70(12): 4360‐4365.
- Androutsopoulos G, Adonakis G, Tsamandas A, et al. Systemic sclerosis and multiple cancers of the female genital tract: prolonged survival following current treatment strategies. Case Rep Rheumatol, 2011; 2011: 392068.
- Androutsopoulos G, Decavalas G. Management of endometrial cancer. Int J Transl Commun Med, 2013; 1: 101.
- Wang T, Zhang X, Lu Z, et al. Comparison and analysis of the clinicopathological features of SCEO and ECOM. J Ovarian Res, 2019; 12(1): 10.
- Makris GM, Manousopoulou G, Battista MJ, et al. Synchronous endometrial and ovarian carcinoma: a case series. Case Rep Oncol, 2017; 10(2): 732‐736.
- Kelemen LE, Rambau PF, Koziak JM, Steed H, Köbel M. Synchronous endometrial and ovarian carcinomas: predictors of risk and associations with survival and tumor expression profiles. Cancer Causes Control, 2017; 28: 447–457.
- Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol, 2001; 83: 355–362.
- 10. Soliman PT, Solmovitz BM, Broaddus RR, et al. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol, 2004; 94: 456-462.
- Scully RE, Young RH, Clement PB, et al. Atlas Pathology, Tumors of the ovary, maldeveloped gonads, Fallopian tube, and broad ligament. 3-rd Series, Fascicle 23. Armed Forces Institute of Pathology. Washington, DC, 1998; 126.
- Mariani A, Sebo TJ, Katzmann JA, et al. HER-2/neu overexpression and hormone dependency in endometrial cancer: analysis of cohort and review of literature, 2005; 25(4): 2921-2927.
- Dordevic M, Mitrovic S, Jovanovic B. Sinhroni tumori uterusa- prikaz slucaja: ed Pregl, 2010; LXIII (7-8): 570-573.
- Halperin R, Zehavi S, Hadas E, et al. Simultaneous carcinoma of the endometrium and ovary vs endometrial carcinoma with ovarian metastases: a clinical and immunohistochemical determination. Int J Gynecol Cancer, 2003; 13 (1): 32-37.
- Karki S, Chapagain U. Synchronous primary tumors of the endometrium and ovary. J Path Nepal, 2012; 2: 189-192.
- Rodolakis A, Thomakos N, Akrivos N, et al. Clinicopathologic insight of simultaneously detected primary endometrial and ovarian carcinomas. Arch Gynecol Obstet, 2012; 285(3): 817-821.
- Falkenberry SS, Steinhoff MM, Gordinier M. et al. Synchronous endometroid tumors of the ovary and endometrium. J Reprod Med, 1996; 41: 713-718.
- Chen Lq, Zhao Q, Lv X. Characteristics and prognosis of coexisting adnexa malignancy with endometrial cancer: a single institution review of 51 cases. Arch Gynecol Obstet, 2011; 283: 1133-1137.
- Montoya F, Martin M, Schneider J, et al. Simultaneous appearance of ovarian and endometrial carcinoma: a therapeutic challenge. Eur J Gynaecol Oncol, 1989; 10: 135–139.
- Chiang YC, Chen CA, Huang CY, et al. Synchronous primary cancers of the endometrium and ovary. Int J Gynecol Cancer, 2008; 18: 159-164.
- Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas - a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol, 2001; 83: 355-362.
- Liu Y, Li J, Jin H, et al. Clinicopathological characteristics of patients with synchronous primary endometrial and ovarian cancers: A review of 43 cases. Oncol Lett, 2013; 5: 267-270.
- Signorelli M, Fruscio R, Lissoni AA, Pirovano C, Perego P, Mangioni C. Synchronous early - stage endometrial and ovarian cancer. Int J Gynaecol Obstet, 2008; 102: 34-38.
- Androutsopoulos G, Adonakis G, Tsamandas A, et al. Systemic sclerosis and multiple cancers of the female genital tract: prolonged survival following current treatment strategies. Case Rep Rheumatol, 2011; 2011: 392068.
- Androutsopoulos G, Decavalas G. Management of endometrial cancer. Int J Transl Commun Med, 2013; 1: 101.
- Androutsopoulos G. Current treatment options in patients with endometrial cancer. J Community Med Health Educ, 2012; 2: e113.
- Khan M, Amin SV, Srinivas SB, et al. Hydrosalpinx as a rare presentation of synchronous ovarian and endometrial carcinoma–a case report. J Clin Diagn Res, 2016; 10(7): QD01‐QD03.
- Shih KK, Yun E, Gardner GJ, et al. Surgical cytoreduction in stage IV endometrioid endometrial carcinoma. Gynecol Oncol, 2011; 122: 608–611.
- Williams MG, Bandera EV, Demissie K, et al. Synchronous primary ovarian and endometrial cancers: a population-based assessment of survival. Obstet Gynecol, 2009; 113: 783–789.
- Dębska‐Szmich S, Czernek U, Krakowska M, et al. Synchronous primary ovarian and endometrial cancers: a series of cases and a review of literature. Prz Menopauzalny, 2014; 13(1): 64‐69.
- National Comprehensive Cancer Network Uterine neoplasms, 2022.
- Ma S, Zhang H, Sun Y, Wu L. Synchronous primary cancers of the endometrium and ovary: review of 43 cases. Chinese - German Journal of Clinical Oncology, 2009; 8: 95-99.
- Heitz F, Amant F, Fotopoulou C, et al. Synchronous ovarian and endometrial cancer - an international multicenter case - control study. Int J Gynecol Cancer, 2014; 24: 54-60.
- Kong A, Nick Johnson, Henry C, Kitchener, et al. Meta-analysis on all stage I endometrial cancer patients who had adjuvant radiotherapy versus no radiotherapy. Ann Oncol, 2007; 18: 1595-1604.
- Kong A, Powelly M, Blake P. The role of postoperative radiotherapy in carcinoma of the endometrium. Clin Oncol, 2008; 20: 457-462.

