Sebaceous Carcinoma of the Breast: What Do We Really Know?
Iva Mitkova Borisova1,*, Lidia Blay Aulina1, Maria Iciar Pascual Miguel1, Paula Rodríguez Martinez2, E M van der Linde3 and Joan Fransesc Julián1
1Department of General Surgery, Hospital Universitari Germans Trias i Pujol, 08916 Badalona, Spain
2Department of Anatomic pathology, Hospital Universitari Germans Trias i Pujol, 08916 Badalona, Spain
3Department of General Surgery, Universitair Medisch Centrum Utrecht, 3584 Utrecht, Nederland
Received Date: 24/04/2023; Published Date: 08/08/2023
*Corresponding author: Iva Mitkova Borisova, MD, Department of General Surgery, Hospital Universitari Germans Trias i Pujol, c. Breda 13, Barcelona 08029, Spain
Abstract
Sebaceous carcinoma of the breast (SBC) is a very rare pattern. Our purpose is to summarize and update the information we have about it till now.
We present all reported cases since 1992 beside our own case and also a review of the literature about its clinical, histological and immunohistochemical features. We analyze its hormonal behavior and prognosis.
According to the World Health Organization (WHO) 5th edition, SCB is no longer considered its own entity but rather a special morphological pattern. There is different hypothesis about its origin, being the most accepted one that the SBC is a consequence of malignant transformation of local ductal cells capable of divergent differentiation. The histology and immunohistochemistry are crucial to distinguish it from others like the glycogen-rich pattern, lipid-rich pattern or the Paget disease. We also investigate the hormonal behavior, concluding that the all-negative subtype is related with the worst survival rates. The AR-positivity could be related with the prognosis as well, but there is a lack of information about this marker in the literature.
Sebaceous carcinoma of the breast is a special morphological pattern that should be included in the differential diagnosis of the breast cancer. Beside the hormonal behavior, other markers as the AR-positivity must be studied to determine better its prognosis.
Keywords: Sebaceous carcinoma; Breast carcinoma; Morphological pattern; Foamy cells
Introduction
The sebaceous carcinoma of the breast (SCB) is a rare type. Currently, according to the World Health Organization (WHO) 5th edition, SCB is no longer considered its own entity but rather a special morphological pattern within invasive breast carcinoma of no special type (IBC-NST) regardless of the extent of differentiation [1].
Case Report
A 57-year-old female with no clinical history of illness presented a palpable lesion in the right breast that started to increase in size quickly over the last 3 months. The physical examination revealed a hard mass, measuring about 10cm and a right axillary lymph node swelling. The computed tomography confirmed the presence of a tumor that occupied the whole right breast and multiple swollen lymph nodes along the level I and II of the right axillary fossae. There were no distant lesions. The thick-needle biopsy of the tumor showed invasive carcinoma with extensive areas of sebaceous differentiation. The fine-needle aspiration cytology of the lymph node showed no malignant cells.
A right radical mastectomy and regional lymph node dissection was considered as surgical treatment after discussion in a multidisciplinary meeting. Macroscopically, the tumor measured 11cm with an ulcerated component on the external surface and in continuity with intraparenchymal solid mass. Histologically, the tumor exhibited a metaplastic spindle cell carcinoma with chondroid differentiation and epithelial component of IBC-NST with sebaceous pattern in its entirety (Figure 1). The sebaceous pattern was composed by lobulated and solid nests of tumor cells with abundant clear and vacuolated cytoplasms and nuclei with intermediate pleomorphism. The metaplastic carcinoma was composed of a diffuse neoplastic proliferation of spindle cells with marked nuclear pleomorphism and focal chondromyxoid differentiation (heterologous elements).
The immunohistochemistry of the specimen showed that both components were positive for p63 and negative for hormone receptors and HER2. The epithelial component was also positive for CK AE1/AE3, CK 5/6 and BerEP4. Ki67-index was around 30%. There was no morphological evidence of metastasis in the axillary nodes. According to the 8ª edition of the TNM staging system, the breast cancer was classified as a pT4b pN0 (stage IIIB).
Postoperatively, she underwent six cycles of chemotherapy (docetaxel + doxorubicin + cyclophosphamide), followed by radiotherapy (total dose 50.0 Gy). She remains free of disease after 50 months of follow-up.

Figure 1: a) The neoplasia consisted of two different tumoral types, on the right a metaplastic spindle cell carcinoma and on the left, a solid epithelial nest with sebaceous pattern (HE, x20). b) The epithelial component showed a prominent sebaceous differentiation characterized by cells with abundant clear and vacuolated cytoplasms (HE, x200). c) Abudant mitotic figures were identified (HE, x200). d) The metaplastic carcinoma was composed of a proliferation of spindle cells with marked nuclear pleomorphism and atypical mitosis (HE, x200). e) Focal chondromyxoid differentiation was observed (HE, x200).
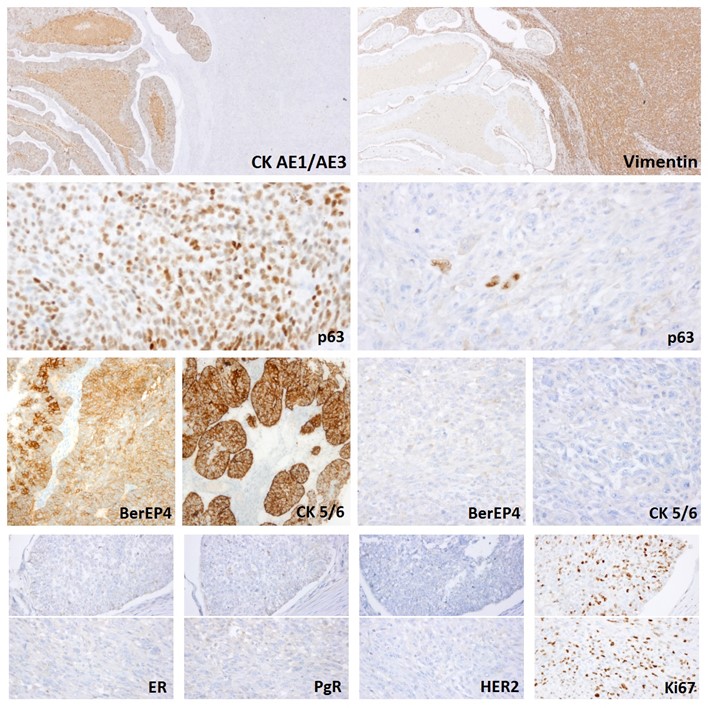
Figure 2: Immunohistochemistry revealed positivity for CK AE1/AE3, BerEP4, CK5/6 in epithelial component, vimentin in sarcomatous component and p63 in both. Breast biomarkers (ER, PgR, HER2) were negative and Ki67-index was around 30%.
Discussion
We analysed all reported cases in Pubmed since 1992 until 2020, beside of our own case, 26 patients in total. We expose the summary of its demographic features, histology, immunohistochemistry, hormonal behavior and prognosis in Table 1.
- Definition:
The majority of the patients suffering from SCB is female with a median age of 60 years [2]. During the physical examination, the lesion is frequently recognized as a palpable mass [3]. Due to insufficient clinical evidence available for their designation as special tumour subtypes, in the last WHO edition (2019) this specific pattern is considered as a part of the spectrum of differentiation seen in IBC-NST [1].
- Origin:
Varga et al. (4), Ohara et al. [5], and Numoto et al. [6] expose hypotheses that explain the origin of SCB, respectively, as follows: (a) The mammary glands of patients with breast cancer may exhibit features of sebaceous glands because these glands share the same origin. (b) SCB develops from progenitor cells capable of differentiation into the sebaceous glands within the epithelium. (c) Sebaceous glands are ectopically present in the mammary gland [7]. However, the presence of in situ ductal carcinoma (DCIS) in some cases supports the theory that sebaceous differentiation in breast neoplasms is unlikely to have origin in putative preexisting ectopic sebaceous glands. It is more likely to be a consequence of malignant transformation of local ductal cells capable of divergent differentiation. Maia et al. propose that in SCB, neoplastic transformation occurs in similar local pluripotent cells that regain capacity to recapitulate pilosebaceous ontogenesis [8]. Furthermore, that could explain the histological variety of components observed in different cases. Squamous differentiation, comedo-like necrosis and metaplastic breast carcinoma have also been reported in some of SCB, the last one also in our own case [2,9,10].
Some authors also suggest considering the diagnosis of SCB in patients with Muir-Torre syndrome, which is characterized by deficiencies of mismatch repair proteins [8]. However, the status of mismatch repair proteins in eleven of the published cases was reported as intact expression [2,8,11].
- Histology:
Sebaceous carcinoma of the breast is a specific pattern, which is part of the spectrum of differentiation seen in IBC-NST, characterized by a lobular or nested growth pattern of tumor cells variably admixed with cells displaying sebaceous differentiation [12]. SCB should be differentiated from other rare morphological patterns of breast carcinoma like glycogen-rich carcinoma, lipid-rich carcinoma, sebaceous adenocarcinoma of the skin and apocrine carcinoma.
SCB should be diagnosed when the tumor has expansive growth and lobular structure, usually displaying the characteristic double-cell population of large, clear, and smaller cells like in Figure 1. One cell population consists of sebaceous gland-like tumor cells, which are mostly located in the center of the lobules or cellular nests. These represent a more differentiated cell type and are rich in vacuolar cytoplasm. The other cell population consists of smaller oval or fusiform non-vacuolar cells, mostly located at the periphery of the lobules. The latter are usually undifferentiated and are difficult to distinguish from typical ductal carcinoma cells [13].
- Immunohistochemistry:
The immunohistochemical stains of sebaceous carcinoma are usually positive for adipophilin (90% of the cells), for epithelial membrane antigen (EMA) and cytokeratins (CK 5/6, CK7, AE1-AE3, CK19, CK20 or CAM 5.2). There were a small number of documented cases with expression of GATA-3, p63, BerEP 4 and no cases with E-cad, S100 and GCDFP-15 expression [8,14]. The GCDFP-15 negativity attests the lack of apocrine differentiation. The EMA and cytokeratin positivity are conformed to the features of sebaceous cells in locations like the skin or ocular adnexa [9,10].
- Hormonal behavior and subtypes:
Based on a close analogy between SCB and ductal carcinoma, Na et al. devised an intrinsic classification system for all types of sebaceous carcinoma. Their study proposes 5 subtypes according to the expression profile of the hormone receptors (HR), HER2 and CK 5/6:
- Luminal 1: HR (+), HER2 (-)
- Luminal 2: HR (+), HER2 (+)
- HER2: HR (-), HER2 (+)
- All-negative: HR (-), HER2 (-), CK 5/6 (-)
- Core Basal: HR (-), HER2 (-), CK 5/6 (+)
Cases harboring HRs, are allotted to the luminal subtypes, in which HER2 positivity distinguishes luminal subtype 2 from luminal subtype 1. The HER2 subtype is defined as HR-negative and HER2-positive. The all-negative and the core basal subtype constitute the triple-negative group, but the last one positive for CK 5/6.
- Prognosis and follow-up:
Na et al. found significant differences in survival rates among the 5 groups with the luminal subtype 2 showing the best survival rates and the all-negative subtype having the worst (p=0.001). Both, the all-negative and core basal subtypes show relatively high treatment failure rates and advanced disease status. Also, tumors in the triple-negative group more commonly were associated with larger tumor size (p=0.001) as in our case. The patients with AR positivity showed a negative association with stage (p<0.001) and treatment failure (p = 0.034) [15].
We discovered a lack of information in the survival rates in a one-third of the patients. One third of this series of 27 patients had axillary lymph node metastases at the moment of the diagnosis and at least 4 patients developed distant metastases [2,16]. Three patients suffering from SCB died because of the disease in 9, 22 and 28 months [2,8,9].
There was a total of 6 patients with triple-negative subtype. CK 5/6 wasn’t registered in none of them, so it wasn’t possible to distinct the core basal subtype. The first one had the peculiarity to suffer from a metaplastic breast carcinoma with 30% of sebaceous differentiation and vertebral metastases at the moment of diagnosis [9]. The metaplastic breast carcinoma is characterized by an aggressive behavior and a poorer prognosis in comparison with invasive ductal carcinoma. This case received radiation therapy targeted to the surgical site and the vertebral lesion, but died 22 months after the diagnosis due to lung metastases. The second triple-negative patient was diagnosed with sebaceous breast carcinoma graded T3N1M0, AR-positive. She had surgery on the breast, but refused chemotherapy and radiotherapy. She developed axillary recurrence and died of widespread dissemination of the disease after 9 months of follow-up [8]. The other case of this triple-negative group developed a local recurrence 10 months after a partial mastectomy [17]. The largest follow-up from the remaining 3 cases of triple-negative type is the one of our own cases. After 50 months of follow-up our patient remains disease-free.
About AR-expression, it is hard to draw conclusions, because we only found 6 AR-positive patients, the half of them without registration of the follow-up. We suggest that this marker should be investigated more in-depth because of its possible correlation with the prognosis.
In this study, the percentage of Ki-67 was generally high (20-30% on average), ranging from 5 to 90%. Moreover, most breast sebaceous carcinoma belong to the luminal subtype, which has a good prognosis. We found 12 well-documented cases of luminal types of SCB. The longest follow-up was 132 months [13].
Table 1: Clinicopathological features of reported sebaceous carcinoma of the breast and breast tumors with sebaceous differentiation.

Abbreviations: AR androgen receptor, AWD alive with disease, AWNED alive with no evidence of disease, CAM 5.2 anti-low molecular weight keratin, CK cytokeratin, D deceased, DOD died of disease, EMA epithelial membrane antibody, ER estrogen receptor, F female, GATA-3 GATA binding protein 3, HER2 human epidermal growth factor receptor type 2, M male, MSI microsatellite instability, Ki-67 PI Ki-67 proliferation index, NA not available, ND not damaged, NS not stated by the reporting author, PgR progesterone receptor, SD sebaceous differentiation
*According to International Union Against Cancer TNM Classification of Malignant Tumors (7th Edition)
** According to the American Joint Committee on Cancer 2018
Conclusion
The sebaceous breast carcinoma is considered nowadays a special morphological pattern. Histologic and immunochemical findings are crucial for its diagnosis. Beside the hormonal behavior, other markers as the AR-positivity must be studied to determine better its prognosis.
Autorship Criteria:
- Iva Mitkova Borisova: drafting the article, integrity of the work
- Lidia Blay Aulina: concept and desing of study; final approval of the version to publish
- Maria Iciar Pascual Miguel: concept and desing of study;
- Paula Rodríguez Martinez: critical revision
- M. van der Linde: critical revision
- Joan Fransesc Julián: final approval of the version to publish
Conflict of Interest: I declare no conflict of interest.
Grant information: The authors received no specific funding for this work.
References
- Rahka EA, Allison KH, Bu H, Ellis IO, Foschini MP, Horii R, et al. Invasive breast carcinoma of no special type. In: Allison KH, Brogi E, Ellis IO, Fox SB, Morris EA, Sahin A, Salgado R, Sapino A, Sasano H, WHO classification of tumours of the breast. 5th. IARC, Lyon: s.n., 2019: 71-76.
- Svajdler M, Baník P, Poliaková K, Straka L, Hríbiková Z, Kinkor Z, et al. Sebaceous carcinoma of the breast: report of foyr cases and review of the literature, 2015; 142-148.
- Hisaoka M, Takamatsu Y, Hirano Y, Maeda H, Hamada T. Sebaceous carcinoma of the breast: case report ans review of the literature. Virchows Arch, 2006; 449: 484-488.
- Varga Z, Kolb SA, Flury R, Burkhard R, Caduff R. Sebaceous carcinoma of the breast. 2000, Pathol Int, 2000; 50: 63-66.
- Ohara N, Taguchi K, Yamamoto M, Nagano T, Akagi T. Sebaceous carcinoma of the submandibular gland with high-grade malignancy: report of a case. Pathol. Int, 1998; 48: 287–291.
- Numoto S, Iwata J, Nakai T, Aki F. A case of sebaceous carcinoma of the breast. Jpn J Diagn Pathol, 2007; 24: 58–61.
- Ohno K, Okada T, Nakamura T, Koyama H. Sebaceous carcinoma of the breast predominantly characterized by intraductal growth: a case report. Surgical Case Reports, 2020; 6: 41.
- Maia T, Amendoiera I. Breast sebaceous carcinoma - a rare entity. Clinico-pathological description of two cases and brief review. Wichows Archiv 2018.
- Carlucci M, Iacobellis M, Colonna F, Mrseglia M, Gambarotti M, Giardina C, et al. Metaplastic Carcinoma of the Breast With Dominant Squamous and Sebaceous Differentiation in the Primary Tumor and Osteochondroid metaplasia in a distant metastasis: Report of a Case with Review of Sebaceous Differentiation in Breast Tumors. International Journal of Surgical Pathology, 2012; 20(3): 284-296.
- Prescott RJ, Eyden BP, Reeve NL. Sebaceous differentiation in a breast carcinoma with ductal, myoepithelial and squamous elements. Histopathology, 1992; 21: 181-184.
- Kinkor Z, Meciarová I, Havlícek F. Primary sebaceous carcinoma of the breast; three casuistic reports. Ceska Gynekol, 2010; 75: 50-53.
- Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma-rare types: review of the literature. Ann Oncol, 2009; 20: 1763-1770.
- Heng C, Wei T, Yingbing T, Hanzhong L. Clinicopathological Characteristics Of breast sebaceous adenocarcinoma. Pol J Pathol, 2018; 69(3): 226-233.
- Sakai Y, Ohta M, Imamura Y. Sebaceous carcinoma of the breast: histological, cytological and ultrastructural features. Breast J, 2018: 1-2.
- Na HY, Choe JY, Shin SA, Choung H, Oh S, Chung J, et al. Proposal of a Provisional Classification of Sebacesous Carcinoma Based on Hormone Receptor Expression and HER2 Status. Am J Surg Pathol, 2016; 40: 1622-1630.
- Yumamoto Y, Nakamura T, Koyama H, Toshiharu K, Moritani S, Ichihara S. Sebaceous carcinoma of the breast: a case report. Surgical Case report, 2017; 3: 38.
- Ramljak V, Sarcevic B, Vrdoljak DV, Kelcec IB, Agai M, Ostovic KT. Fine needle aspiration cytology in diagnosing rare breast carcinoma--two case reports. Coll Antropol, 2010; 34: 201–205.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical straining for androgen receptors: a sensitve marker of sebaceous differentiation. Am J Dermatopathol, 1999; 21: 426-431.
- Kariya Y, Moriya T, Suzuki T, Chiba M, Ishida K, Takeyama J. Sex steroid hormone receptors in human skin appendages and its neoplasm. Endocrine J, 2005; 52: 317-325.
- Thiboutot D, Gilliland K, Light J. Androgen metabolism in sebaceous glands from subjects with and without acne.. Arch Dematol, 1999; 135: 1041-1045.
- Lara B. Carcinoma sebaceo de la mama. Rev Esp Patol, 2016.
- Acosta A, Mohamed R, Harron al Rasheed, H Xu, C Salibay, M Pins. Sebaceous carcinoma of the breast in a patient with a pathogenic BRCA2 (886delGT) mutation – focus on histopathologic and immunohistochemical features. APMIS, 2018; 126: 353-356.

