Coarctation of the Aorta Revealed about a Dilated Cardiomyopathy: Late and Unusual Diagnosis in a Thirty-Something Patient
Hamissou I*, Nacocan D, Guedira M, Lemzabi Y, Cherti M and Amri R
Department of Cardiology B, CHU Ibn Sina, Mohammed V University, Rabat, Morocco
Received Date: 17/04/2023; Published Date: 27/07/2023
*Corresponding author: Ibrahim O Hamissou, Department of Cardiology B, CHU Ibn Sina, Mohammed V University,Rabat, Morocco
Abstract
Coarctation of the aorta is a rare congenital heart disease (5-8%) whose etiology is not clearly elucidated, based on two hypotheses. Symptoms and clinical signs vary according to age and the degree of stenosis of the coarcted segment in children, while in adults we find in foreground hypertension in the upper limbs. The TTE confirms the diagnosis. The thoracic scanner is fundamental before a therapeutic decision. Endovascular treatment is encouraged over surgery with indications well codified by the European Society of Cardiology (ESC).
We offer you, at the Cardiology B department in Rabat, an unusual diagnostic scenario in one of our thirty-year-old patient without a cardiovascular history, in whom, starting from a DCM assessment, we were able to bounce back on a coarctation of the isthmic aorta associated with a type 0 bicuspid aortic valve.This case, in addition to the clinical semiology that it allowed us to describe, offers us a rich iconography in terms of ultrasound (five images) and scanner (three images) of the coarctation of the aorta.
Finally, it was the opportunity to discuss the latest recommendations of the ESC 2020
Keywords: Coarctation; Coarctation of the aorta; Cardiomyopathy; Dilated cardiomyopathy
Abbreviations: 1st AVB: First degree atrioventricular block; AD: Arterial duct; AR: Aortic Regurgitation; BP: Blood pressure; CIV: Inter-ventricular communication; COA: Coarctation of the aorta; DCM: Dilated Cardiomyopathy; EROA: Effective Regurgitant Orifice Area; ESC: European Society of Cardiology; G: gradient; LAFB: Left Anterior fascicular block; LVEF: Left Ventricular Ejection Fraction; LVH: Left Ventricular Hypertrophy; LV: Left ventricle; Re-CoA recoarctation; RV: Regurgitant Volume; SCA: Subclavian Artery; TEE: Trans-esophageal ultrasound; TTE: Transthoracic ultrasound
Introduction
Coarctation of the aorta is a congenital narrowing of the aortic isthmus. It concerns 5-8% of congenital heart diseases with a male predominance (sex ratio to 2H/1F) [1].
The coarctation can be isolated or associated with other abnormalities such as bicuspid aortic valves found in 50% of cases.
There are two etiological hypotheses of coarctation of the aorta: the ductal theory and the hemodynamic theory. [2].
Signs and symptoms depend on age and severity of coarctation. Before birth, prenatal diagnosis is based on the presence of asymmetry in the size of both ventricles in the second semester of pregnancy. After birth, three situations are possible: the very severe form in the newborn who presents with acute heart failure; the long-tolerated severe to moderate form in children and adults which manifests as arterial hypertension in the upper limbs with weak or absent pulses in the lower limbs [3].
The TTE makes it possible to show the stenosis and to evaluate its impact on the heart. CT angiography and angio-MRI are necessary as part of the preoperative assessment [4] Treatment is surgical in symptomatic children from three months of age.
In adults, the indication for surgery by endovascular treatment (stenting) is first-line recommendation in the presence of hypertension with a gradient >20 mmhg between the upper and lower limbs [2,5].
We report the case of one of our patients from the cardiology department B of the Ibn Sina hospital in Rabat, thirty nine years old, without cardiovascular risk factors and without medical history, admitted for cardiac decompensation.
We performed an unusual diagnostic scenario in this patient, starting from the discovery of a DMC on echocardiography to rebound on a coarctation of the aorta seen late with a LV unsuited to the post load . This coarctation was associated with a bicuspid aortic valve.
Case Report
This is a 39-year-old patient, married and mother of 03 children, not known to be hypertensive, with no other cardiovascular risk factors and no medical history. The patient experienced rapidly worsening dyspnea (after her last pregnancy) related to primary cardiac decompensation, which led to her admission to a peripheral hospital (in Kenitra) where she was balanced . After hemodynamic stabilization, she was referred to the UHs in our department for additional care.
Clinical examination on admission found sinus tachycardia at 100 bpm,blood pressure in both upper limbs was normal at 120/60 mmhg.Cardiac auscultation revealed a moderate murmur of aortic stenosis,aortic regurgitation and mitral regurgitation.
The ECG (Figure 1) showed sinus rhythm, 1st degree AV block, electrical Left Atrium enlargement and electrical LVH on Comeil index at 28 mm.
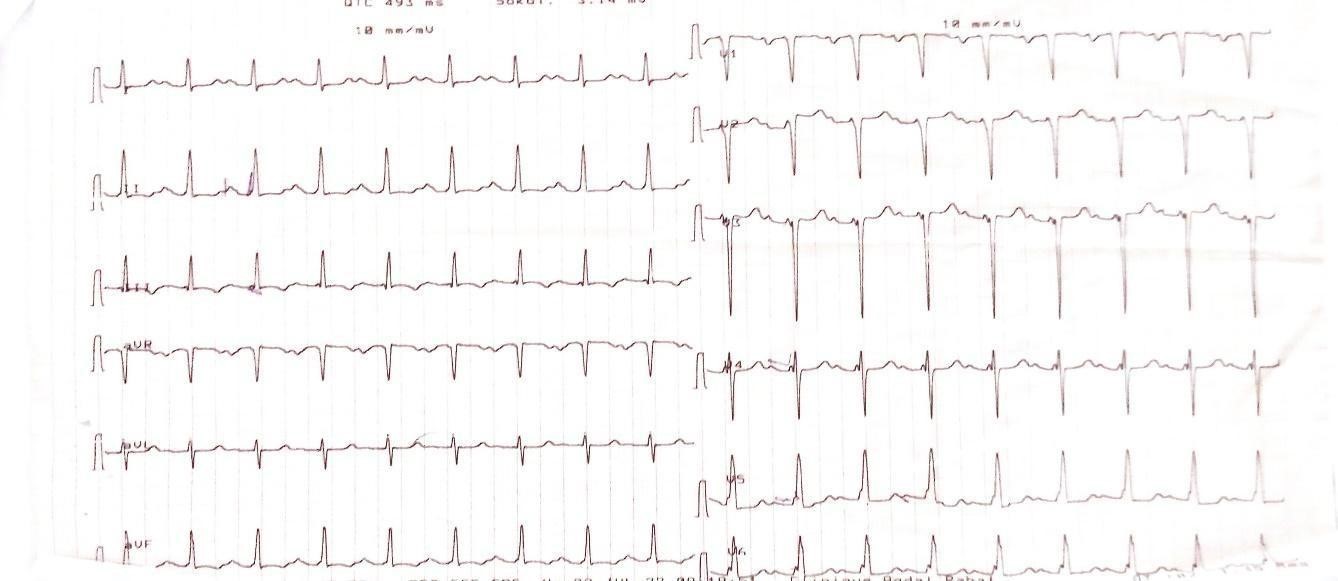
Figure 1: ECG showing electrical LVH with Comell index at 28mm.
Transthoracic ultrasound revealed DCM with an LV with hypertrophied walls, globally hypokinetic and unsuited to afterload (LVEF at 35%).
However, there was no aortic stenosis explaining the LVH (in a normo-tense patient). This led us to make a suprasternal window which showed a coarctation of the isthmic aorta (Figure 2, 3) located after the left subclavian artery, the velocity in the portion of the narrowed aorta was 4.17m/s (Figure 4).
The flow in the abdominal aorta was non-pulsating (Figure 5, 6).
On transthoracic Doppler there was a moderate AR (RV= 15ml and EROA= 17mm2) which explored at TEE was associated with bicuspid aortic valve type 0.

Figure 2 and 3: Coarctation of the aortic isthmus.
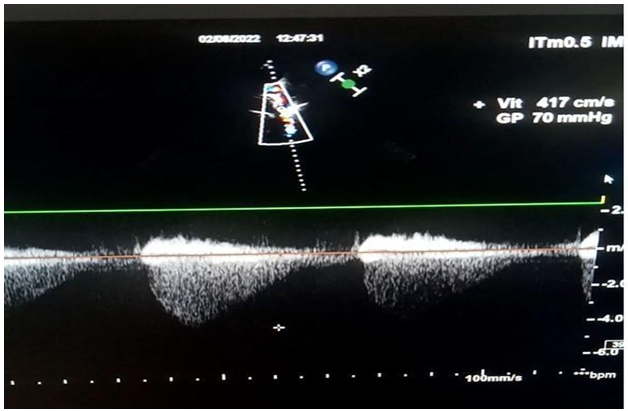
Figure 4: Trans-stenotic Vmax at 4.17m/s with G= 70mmhg.

Figure 5: Non-pulsed Doppler in the abdominal Aorta.
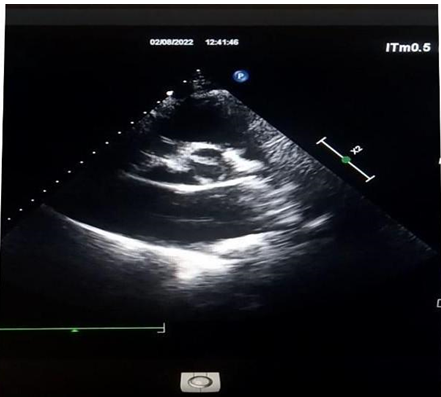
Figure 6: Bicuspidy type O.
On re-examining the patient, the blood pressure in the lower limbs was 80/50mmhg (generating a gradient of about 30mmhg between upper and lower limbs).
Femoral pulses were absent bilaterally.
As part of the pre-therapeutic assessment, we performed a CT angiography which shows a short and tight coarctation of the aorta, with an isthmic seat 30mm from the birth of the left subclavian artery (Figure 7).
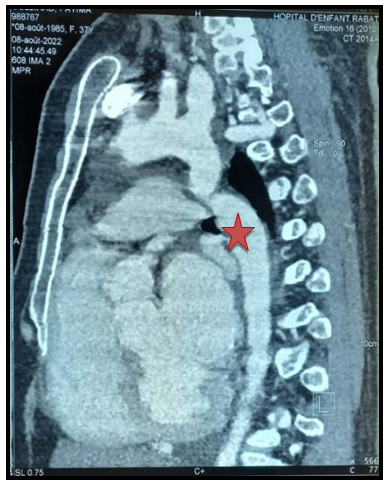
Figure 7: Stenosis of the aortic isthmus at 30mm after the birth of the subclavian artery(Red star).
Prevertebral, internal mammary and intercostal collateral circulations developed, the largest one which is the left prevertebral measured 15mm (Figure 8, 9).

Figure 8 and 9: Prevertebral collateral arterial circulation (red stars).
A CT scan of the abdomen described a suspicious inferior polar mass in the right kidney
Note that on the chest X-ray there was lysis at the level of the lower part of the ribs related to indirect signs of the presence of intercostal collateral venous circulation (Figure 10).

Figure 10: lysis at the level of the lower part of the ribs.
The standard biological assessment was normal with negative viral serologies (HIV, HBV, HCV).
Discussion
Coarctation of the aorta is a congenital stenosis of the aorta most often affecting the segment after the departure of the left subclavian artery.
It represents between 5 to 8% of congenital heart diseases. Rarely isolated, it is most often associated with other heart defects as was the case in our patient with a bicuspid aortic valve. This association between CoA and bicuspid valve is the most frequent, found in 50-85% of cases.
Hypoplasia of the aortic arch can be found in 30% of cases.
Two theories are predominant on the etiology of the CoA. The first is that of the ductal tissue. CoA would develop due to the migration of cells from the ductus arteriosus into the periductal aorta, resulting in narrowing upon closure of the canal. This is confirmed by the fact that the administration of prostaglandins in the newborn makes it possible to reopen the ductus, but also to temporarily lift the coarctation. The second theory is hemodynamic. In a normal fetus, only 10% of cardiac output crosses the aortic isthmus. In case of associated left heart anomalies (ventricular septal defect(VSD), aortic stenosis, etc.), the flow in the ascending aorta will be reduced and there by reduced the growth of the aortic arch, mainly at the level of the isthmus and promotes CoA [2]
Signs and symptoms are age dependent. The prenatal diagnosis is made in front of the asymmetry of size of the two ventricles, the presence of a left superior vena cava also can make suspect the presence of a CoA. In the newborn two situations can arise: a severe form with an acute heart failure and differential cyanosis; or a moderate form with moderate symptoms. In children and adults, the main sign is hypertension in the upper limbs. weak or non-present femoral pulses, and an interscapular murmur which may be absent in the case of cardiac dysfunction [3].
Echocardiography establishes the diagnosis, shows the site of the COA, evaluates the haemodynamic impact and even allows to look for other cardiac abnormalities. There stenosis is said to be significant when the trans-stenotic gradient on pulsed Doppler is > 20mmhg or >10mmhg in case of LV dysfunction or in case of significant collateral arteries. Our patient despite of her normal BP and LV dysfunction had a trans-stenotic gradient >20mmgh [4].
Note that the ECG is not very contributive, most often showing LVH or even can be normal, especially in newborns.
CT angiography and MRI allow us to detail the anatomy of the aorta. These are necessary pre-therapeutic examinations, also making it possible to show the existence of collateral circulation [4].
Rare in practice but catheterization is the gold standard for measuring the peak-to-peak gradient which is significant if it is 20 mmhg; it allows complete hemodynamic exploration. A aortography is performed in the same time to quantify the severity of the stenosis [2].
The goal of CoA treatment is to remove the obstruction. In children it is surgical, consisting of an end-to-end resection anastomosis called the CARFOORD operation, this is the reference technique for left thoracotomy. There are other surgical means, as extended resection with end-to-end anastomosis, aortoplasty by prosthetic patch,aortoplasty by clamping the left SCA and extra-anatomic bypasses. In adults, endovascular treatment by balloon dilation and stenting is ideal in the case of native CoA or Re-CoA [2].
The indication for intervention in the event of CoA or Re-CoA is well codified by the ESC, it is a class I recommendation for intervention (surgery or stenting) in the case of hypertension with a peak-to-peak gradient >20 mmhg. A class lla recommendation for intervention in patients with hypertension and >50% stenosis even if the peak-to-peak gradient is < 20 mmHg and similarly for patients with normal blood pressure but with a peak-to-peak gradient >20 mmHg. Finally it is a class lIb recommendation for patients with normal blood pressure and < 20mmhg peak-to-peak gradient but >50% of stenosis [5].

Figure 11,12 and 13: ESC 2020 algorithm and guidelines.
Even after treatment, patients are at risk of having complications: residual hypertension. cerebral aneurysms, re-CoA, thus requiring follow-up with a congenital cardiologist.It should be noted that in the absence of treatment, the disease naturally progresses to heart failure, as was the case with our patient. And 75% of patients die by the age of 43 mostly from this same complication.
Conclusion
Coarctation of the aorta is a congenital pathology which is sometimes discovered late in adults during an hypertension check-up. Diagnosis is made by cardiac Doppler ultrasound. In the latest recommendations, endovascular treatment by stenting is the reference treatment, it is preferable to surgery, precisely in the case of native CoA or re-CoA In adults In the absence of treatment, the evolution is naturally towards heart failure. The case reported in our observation is rare, due to the discovery of an association of CoA and a bicuspid aortic valve in a woman with DMC with normal BP and for whom it was necessary to find an explanation for the walls of her LV that were hypertrophied.
References
- Hoffman JI. The challenge in diagnosing coarctation of the aorta. 2018 Jul/Aug 23 Cardiovasc J Afr, 29(4): 252.
- Raymond Pfister. La coarctation aortique » .07.09.2022. Forum Med Suisse, 2022; 22(36): 600-603.
- Familiari A, Morlando M, Khalil A, Sonesson SE, Scala C, Rizzo G, et al. Risk Factors for Coarctation of the Aorta on Prenatal Ultrasound: A Systematic Review and Meta-Analysis. Circulation, 2017; 135: 772–785.
- 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines.
- 2020 ESC Guidelines for the management of adult congenital heart disease | European Heart Journal | Oxford Academic.

