Initial Experience with the Surfacer Device for Intrathoracic Outflow Occlusion: Mid-Term Results
Chris Durham, Hiue Pham and Karl A Illig*
FLOW Vascular Institute Comprehensive Dialysis Access Center, USA
Received Date: 15/04/2023; Published Date: 26/07/2023
*Corresponding author: Karl A Illig, MD, FLOW Vascular Institute Comprehensive Dialysis Access Center, 4301 Vista Rd, Suite 109, Pasadena, TX 77504, USA
Abstract
Objectives: Within the past few years a device (Surfacer® Inside-Out® Access Catheter System, Bluegrass Vascular Technologies, Inc., San Antonio, TX) has become available to restore atrial access from the right neck via an “inside out” approach. While this leaves a dialysis catheter in place, most surgeons follow this with conversion to a HeRO graft, either immediately or in a staged fashion. We report our first years’ experience with this approach in a cohort of patients with intrathoracic venous occlusion.
Methods: Our Electronic Medical Record (EMR) was queried to capture all Surfacer cases done since we began our current practice. Both practice and hospital EMRs were then reviewed to gather needed data. As all data were deidentified, this study was granted a waiver of HIPPA authorization from the WCG Institutional Review Board.
Results: From 2/25/21 to 6/3/22 (17 months) a total of 15 patients underwent 17 Surfacer procedures (mean age 52±15 (range 28 to 85) years old). All patients were dialysis dependent, 11 via femoral catheters, one via a leg AVG, and three via patent upper extremity Arteriovenous Fistulas (AVFs), all three of these with SVC syndrome. One patient had a tracheostomy and one a healing catheter exit site infection on the right, and two patients underwent redo procedures after inadvertent catheter removal. No complications occurred during any of the 17 procedures. A learning curve seemed to be present based on operating room and radiographic times. 12 of the 15 patients proceeded to HeRO graft placement (one has persistently refused and remains catheter dependent and two are awaiting conversion) at a mean of 49±51 (16-189) days later; excluding two patients with long delays 7 had their HeROs at 29.6±15.8 days following Surfacer catheter placement. Again, no complications occurred. At mean followup of 176±133 (range24 to 452) days, all 12 patients are being dialyzed via their HeRO graft and are doing well.
Conclusions: We have had no procedural complications and 100% procedural success in 17 Surfacer cases (and 12 subsequent HeROs) performed in 15 patients over the past year. Redo Surfacer cases are feasible. Both procedural and mid-term results seem favorable, especially given this very disadvantaged group of patients.
Introduction
Any running blood access depends upon unobstructed venous outflow to the heart. While venous obstruction in the extremities is usually fairly easy to treat, intrathoracic venous stenosis and occlusion (defined here as central to the costoclavicular junction) can be much more challenging. If a patent but stenotic lesion is present, conventional techniques are usually adequate. Total occlusions, however, especially if chronic, can be much more challenging, although a variety of techniques are used [1]. Within the past few years, a device (Surfacer® Inside-Out® Access Catheter System, Bluegrass Vascular Technologies, Inc., San Antonio, TX) has become available to restore atrial access from the neck via an “inside out” approach from the right femoral vein. While this leaves a dialysis catheter in place, most surgeons follow this with conversion to a HeRO graft, either immediately or in a staged fashion. We report our initial experience with this approach in a cohort of patients with intrathoracic venous occlusion.
Methods
Our prospectively maintained database, personal records, and Electronic Medical Record (EMR) were reviewed for all Surfacer cases done since we began using this device. Both office and hospital records were reviewed, including details of the operation itself. As all data were deidentified, this study was granted a waiver of HIPPA authorization from the WCG Institutional Review Board. Descriptive statistics only are reported as there is no comparison group, and unless otherwise specified are reported as means ± standard error along with ranges. Bluegrass Vascular reviewed this manuscript for legal and compliance assurance but had no control over data, contents, or results.
Results
From February 2021 to June 2022 (17 months) a total of 15 patients underwent 17 Surfacer procedures (two patients had a second Surfacer procedure after their original catheters had been removed, one for infection and one inadvertently). Mean age of the 15 patients was 52±15 (28 to 85) years old and mean body mass index (BMI) 28.5±8.6 (17.9 to 52.6). All patients were dialysis dependent, 11 via femoral catheters, one via a leg AVG, and three via patent upper extremity AVFs with SVC syndrome. One patient had a tracheostomy and one a healing catheter exit site infection on the right.
For the 17 procedures performed, mean operating room (OR) time was 38.6±9.1 (28 to 63) minutes and mean X-ray time was 360±146 (172-717) seconds. There was a modest correlation between BMI and OR time (r=0.55) but less so for X-ray time (r=0.27). A learning curve was apparent – mean OR time was 48 minutes for the first five cases, 37 minutes for the middle 6, and 36 minutes for the last 6, with X-ray times dropping in a similar fashion (491, 349, and 283 seconds, respectively).
No complications occurred during any of the 17 procedures and all procedures were completed as planned (technical success rate 100%).
12 of the 15 patients have gone on to HeRO graft placement, all successful (one has refused and the other two are awaiting operation). HeRO grafts were placed a mean of 49±51 (16-189) days; excluding two patients with long delays 10 had their HeROs at 29.6±15.8 days following Surfacer catheter placement. Again, no complications occurred. At mean followup of 176±133 (range24 to 452) days, two episodes of thrombosis have occurred and one required Distal Revascularization/Interval Ligation (DRIL) for Hemodialysis Access-Induced Distal Ischemia (HAIDI). All 12 are still being dialyzed via their HeRO graft and are doing well.
Discussion
Any running blood access depends entirely upon unobstructed venous outflow to the heart. Venous outflow of an Arteriovenous (AV) access can be obstructed at various levels, including the access outflow segment itself, the venous anastomosis of an Arteriovenous Graft (AVG), the swing segment of a Basilic Vein Transposition (BVT), or the cephalic arch peripherally. Outflow can also be obstructed more centrally. In the past two decades it has been increasingly recognized that the subclavian vein can be obstructed at the venous thoracic outlet, which most feel must be treated with bony decompression for lasting relief.
What of problems that occur even more centrally, in the chest? Venous anatomy in the chest is shown in Figure 1. On both sides the subclavian veins are joined by the internal jugular veins to create the brachiocephalic veins. Longer on the left, the brachiocephalic veins then merge to form the superior vena cava. Each of these vessels can be affected by various issues inherent in the provision of hemodialysis access (catheters and high flow) or other related problems (pacemaker leads, chemotherapy ports, and misadventure during cardiac surgery, for example), not forgetting natural anatomic variants producing extrinsic compression.
If one side only is obstructed, it may be most prudent to simply move to the other side. This gives up access options, however, and often aggressive attempts at correcting the problem are worthwhile to prolong use of the arm [1]. This topic is especially important today (2023), as several techniques and devices have recently become available to solve these problems.
In the early 2000s, John Gurley, an interventional cardiologist working in Kentucky, used the principle that the veins are anterior to the arteries (and brachial plexus) to create access by pushing a wire from the SVC (via the groin) through the skin of the neck superior to the clavicle. Termed “inside out,” this technique was first used to place pacemaker wires, but it was very quickly realized that catheter placement was the obvious next step [2,3]. Within the past few years, a commercial device (Surfacer System, Bluegrass Vascular Technologies, Inc., San Antonio, TX) has become available to very easily accomplish this. While this leaves a dialysis catheter in place, most surgeons follow this with conversion to a HeRO graft, either immediately or in a staged fashion [4,5].
The Surfacer device (Figure 2) “automates” this process in two ways. First, by means of a specially formed tip, it is advanced through the occluded SVC/right brachiocephalic system for several centimeters. Second, based on the degree of cranio-caudal imaging angulation required, a calibrated pre-shaped Nitinol needle guide is deployed to “point” the sharpened stiff wire toward a target previously placed on the skin. The wire is advanced by means of a plunger system, and if the equipment is lined up properly nothing seems to be happening – you are “staring down the barrel of the gun,” with the wire advancing directly toward the image intensifier – until the wire tents up the skin and ultimately pokes through it (https://bluegrassvascular.com/surfacer/). One drawback of this system is that the device is quite stiff, and thus only right femoral access with right sided neck exit is possible.
The “inside out” procedure (and several variants of recanalization above) are techniques for placing a dialysis catheter. In almost all cases this can be followed by HeRO graft placement. It is our sense that this began as a single-step procedure, but data emerged supporting the observation that the infection rate was lower if access and HeRO graft placement were staged [4]. In addition, we feel best results are obtained with the Surfacer device with the arms tucked to allow full tube rotation, and at least psychologically these are two non-trivial procedures. For all these reasons we favor staging the HeRO by two weeks or so, and using this technique have experienced an acute infection rate of only 2% [5]. As the HeRO uses the catheter tract for atrial access, of course, an immediate access graft must be used (or a femoral catheter placed).
As of June 2022, there have been a total of 12 studies describing outcomes in 124 patients (with seven undergoing a redo procedure). Results from the three “large” multicenter series (30, 30, and 32 patients) show a technical success rate of 95% and no technical complications [6-8]. Our single center results are quite similar. In 15 patients undergoing 17 procedures, our technical success rate of 100% and absence of complications adds further support to the safety and efficacy of this device. Further, redo Surfacer procedures appear to be quite feasible – in our two and the seven in one of the multicenter studies [7], no complications have occurred and technical success was again 100%.

Figure1: Anatomy of the intrathoracic veins. Note the relatively straight pathway from the vena cava to the right innominate and jugular veins. Reprinted from Illig KA, Scher L, Aruny J, Ross JR (eds): Textbook of Dialysis Access. London, Springer, in press.
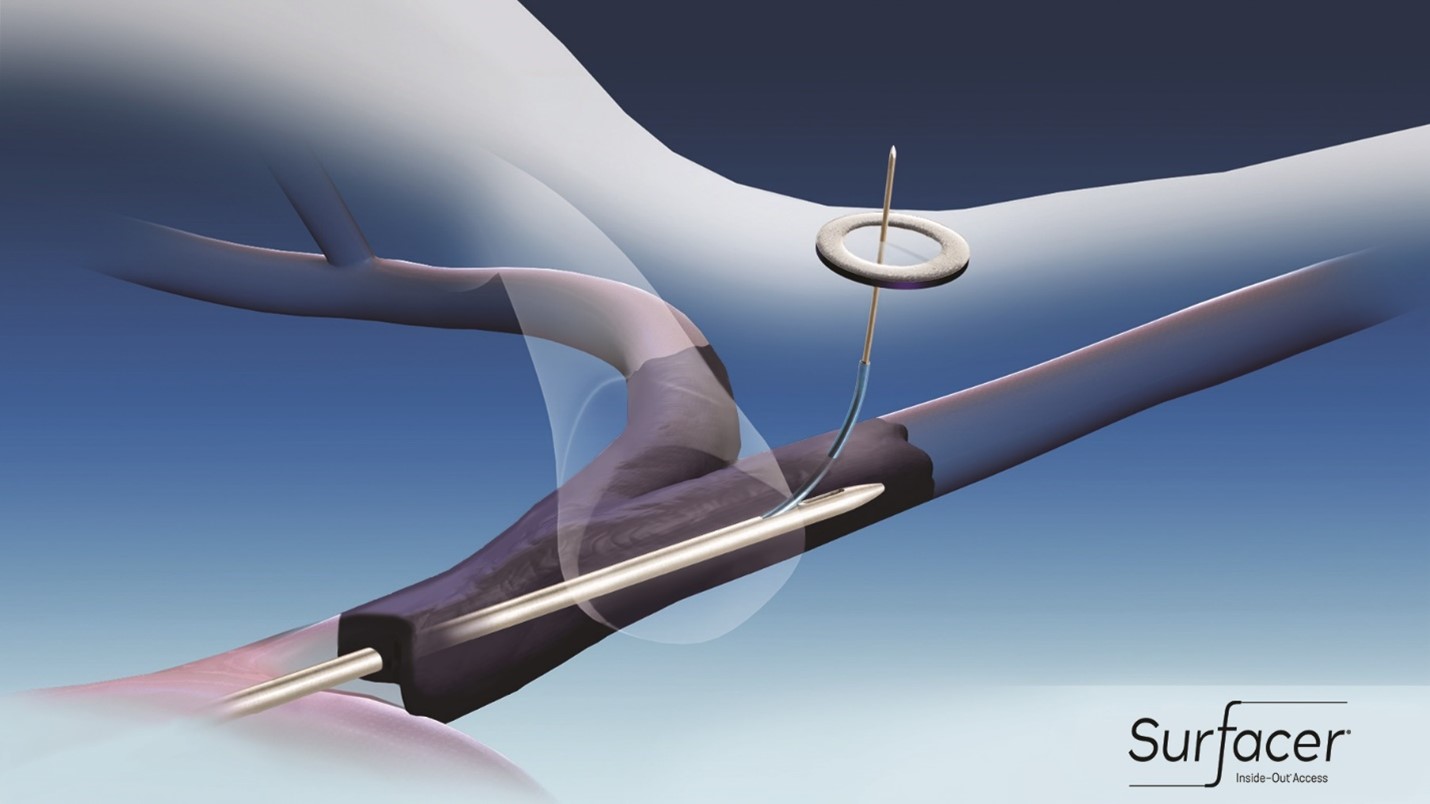
Figure 2: The Surfacer® Inside-Out® Access Catheter System (Bluegrass Vascular Technologies (BVT), San Antonio, TX) is advanced through the complete right brachiocephalic vein and/or right internal jugular occlusion. The needle guide has been extended to the proper angle (based on the amount of craniocaudal tube angulation needed) and the needle wire is advanced to exit target on the skin. Original figure Courtesy BVT.
Conclusion
We have had no procedural complications and 100% procedural success in 17 Surfacer cases (including two redos) and 14 subsequent HeRO cases performed in over the past 17 months. Both procedural and mid-term results seem favorable, especially given this very disadvantaged group of patients.
References
- Illig KA, Kim C. Intrathoracic venous stenosis and occlusion. In Illig KA, Scher L, Ross JR, Aruny J (eds): Principles of Dialysis Access. Springer, in press.
- Elayi CS, Allen CL, Leung S, Lusher S, Morales GX, Wiisanen M, et al. Inside-out access: A new method of lead placement for patients with central venous occlusions. Heart Rhythm, 2011; 8(6): 851-857.
- Ebner A, Gallo S, Cetraro C, Gurley J, Minarsch L. Inside-out upper body venous access. Endovascular Today, 2013; pp.85–89.
- Griffin AS, Gage SM, Lawson JH, Kim CY. Early infection risk with primary versus staged Hemodialysis Reliable Outflow (HeRO) graft implantation, J Vasc Surg, 2017; 65(1): 136-141.
- Illig KA, London MJ, Aruny J, Ross JR. Safe and effective HeRO graft placement: techniques and results. Journal of Vascular Access, 2022; 23(5): 805-812.
- Gallieni M, Matoussevitch V, Steinke T, Ebner A, Brunkwall C, Cariati M, et al: Multicenter experience with the Surfacer inside-out access catheter system in patients with thoracic venous obstruction: Results from the SAVE registry. J Vasc Interv Radiol, 2020; 31(10): 1654-1660.
- Reindl-Schwaighover R, Matoussevitch V, Winnicki W, Kalmykov E, Gilbert J, Matzek W, et al. A novel inside-out access approach for hemodialysis catheter placement in patients with thoracic central vein occlusion. Am J Kidney Dis, 2020; 75(4): 480-487.
- Razavi MK, Peden EK, Sorial E, Ross JR, Aruny JE, Pflederer TA, et al. Efficiency and safety associated with the use of the Surfacer inside-out access catheter system: Results from a prospective, multicenter Food and Drug Administration-approved investigational device exemption study. J Vasc Access, 2021; 22(1): 141-146.

