Dentinogenic Ghost cell Tumour of the Maxilla
Timothy Manzie1, Michael J L Hurrell2,3,*, Jeremy Rawlins4 and Peter Ricciardo5
1Department of Head and Neck Surgery, Oral and Maxillofacial Surgery, Chris O’Brien Lifehouse, New South Wales, Australia
2Maxillofacial Surgery Unit, Gold Coast Hospital and Health Service, Gold Coast University Hospital, Queensland, Australia
3School of Medicine and Dentistry, Griffith University, Queensland, Australia
4Plastics and Reconstructive Surgery, Royal Perth Hospital, Western Australia, Australia
5Oral and Maxillofacial Surgery, Royal Perth Hospital, Western Australia, Australia
Received Date: 04/04/2023; Published Date: 11/07/2023
*Corresponding author: Dr. Michael J L Hurrell, Maxillofacial Surgery Unit, Gold Coast Hospital and Health Service, Gold Coast University Hospital, Queensland, Australia
Abstract
A Dentinogenic Ghost Cell Tumour (DGCT) is an uncommon odontogenic tumour. Rarely, these tumours can undergo malignant transformation. There are a limited number of reported cases with no consensus on appropriate management. While different treatments have been considered, surgical removal with a resective margin is the preferred treatment modality. This case demonstrates management of a previously neglected DGCT of the left maxilla with free flap reconstruction.
Keywords: Dentinogenic; Ghost cell; Tumour; Maxilla; Resection; Free flap
Introduction
Dentinogenic Ghost Cell Tumours (DGCT) are a rare odontogenic benign neoplasm [1]. Initially called a calcifying odontogenic cyst (COC), the lesion was subsequently described as its own entity in the most recent World Health Organisation classification [1,2]. The incidence is unknown with under 50 reported cases worldwide, demonstrating an Asian male preponderance [1]. Surgical enucleation results in a high recurrence rate thus many suggest more aggressive management. This article aims to provide an overview of the currently available evidence. In addition, we present a novel method of reconstruction for a complex maxillary defect following resection of a large DGCT.
Case Description
A 56-year-old Caucasian male presented with a 15-year history of a slowly expanding mass involving the left cheek and hard palate. Examination revealed a large, deforming, bony-hard mass on the left midface, abutting the nose, infraorbital region and extending to the malar prominence. Skin was not involved. Cranial nerves five and seven were intact, with no malposition of the globe and normal ocular movement. No palpable parotid or neck masses were identified. Intraorally, there was a large fungating lesion involving the left hard palate and extending across the midline. The dentition was in poor condition. Speech, swallow, olfaction, tongue movements and mouth opening were all normal. The patient had a history of schizophrenia, but did not take any regular medications. He smoked 5 cigarettes per day, and denied alcohol or other illicit drug use.
Clinical photographs, dental study models and an orthopantomogram were taken. A computed tomography (CT; Figure 1) scan of the facial skeleton and neck, as well as a CT angiogram of the lower limbs was acquired for surgical planning. This demonstrated a 51 x 51 x 73mm expansile mass arising from the left maxilla, with effacement of the maxillary antrum and left nasal passage. Multiple large cystic spaces were also evident within the lesion. The salivary glands, thyroid and cervical lymph nodes all appeared within normal limits. Angiography demonstrated patency of the lower limb vessels (three vessel runoff), confirming the availability for harvest of a fibula free flap for maxillary reconstruction.
A transoral, incisional biopsy was undertaken and demonstrated the presence of ghost cells, dentinoid formation, multifocal calcifications and ameloblastic epithelium with partial cystic change, favouring a DGCT.
Resection was planned with 5mm macroscopic bony margins and supraperiosteal dissection. Following submental intubation, a left sided Weber-Ferguson incision was used to gain access to the ethmoid bone and posterior maxilla. A full dental clearance was undertaken prior to pathological resection. The anterior aspect of the left orbital floor was included in the resection, with sacrifice of the infraorbital nerve distally, but extension beyond the anterior aspect of the inferior orbital fissure was deemed unnecessary; orbital floor reconstruction was not undertaken. The nasal septum was largely preserved, but the entire left hard palate and premaxilla (extending to include the contralateral right canine tooth) was included in the resective margin, along with the left lateral nasal wall. The majority of the soft palate was preserved. The resultant ablative defect was classified as a Brown’s class IIId [3]. The left nasolacrimal duct was transacted and directed toward the nasal cavity. The left medial canthal tendon attachment was released to allow for adaptation of a custom cutting and drilling guide and definitive 3D printed Depuy-Synthes (Massachusetts, United States of America) custom titanium reconstruction plate. The tendon was later reconstructed with a canthopexy barb and tensioned and directed through a 0.8mm miniplate cantilevered from the left frontal bone. The right fibula was utilised for reconstruction, with microvascular anastomosis of the peroneal vessels to the left facial vessels via the trans-facial approach. Four bony segments were utilised, with removal of an intervening segment between the second and third segments to allow for double-barrelling at the level of the zygoma (Figure 2). Adequate bony reconstruction of the alveolus and anterior maxilla, including the infraorbital rim and the majority of the pyriform aperture was achievable. This allowed for restitution of facial contours and minimal disruption to orbital and nasal aesthetics, whilst making future fixed dental rehabilitation a viable option. A soft tissue skin paddle with the fibula allowed for reconstruction of the palatal defect. No significant complications arose in the post-operative period. The patient has had follow-up over 18 months from the time of treatment without evidence of recurrence.
Final histopathological assessment (Figure 3-5) confirmed the diagnosis of DGCT, with clear margins. The patient has recently completed one-year post-operative review, without clinical or radiological evidence of recurrence.
The procedures followed were in accordance with the ethical standards of the treating hospital and in keeping with the Helsinki Declaration (2000). Consent has been obtained from the patient.
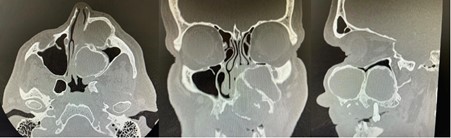
Figure 1: CT scan multiplanar reformation with axial (left), coronal (central) and sagittal (right) slices demonstrating a large, cystic tumour of the left maxilla.

Figure 2: pre-surgical virtual surgical plan (left) and post-surgical CT scan (right) demonstrating accuracy of reconstruction with fibula free flap and 3D printed custom hardware.

Figure 3: Histopathological image of the resective specimen demonstrating cystic and solid components (H&E Stain, 40x magnification).

Figure 4: Histopathological image of the lesion demonstrating the cystic component lined by ghost cells with dentinoid material (H&E Stain, 100x magnification).
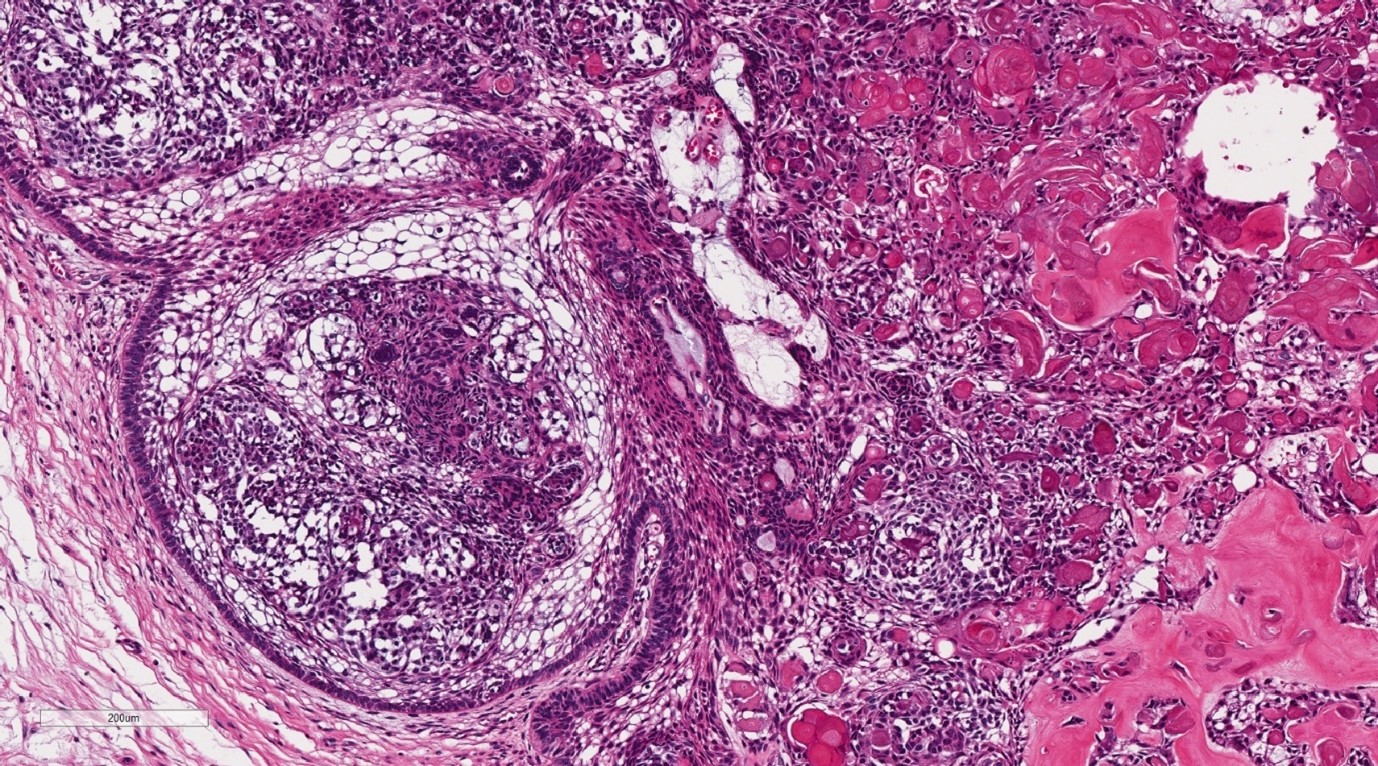
Figure 5: Histopathological image of the lesion demonstrating the solid component with stellate reticulum resembling ameloblastoma with dystrophic calcification (H&E Stain, 100x magnification).
Discussion
Dentinogenic ghost cell tumours are a locally aggressive, rare neoplasm. It may be found in isolation or associated with an odontoma, ameloblastoma and ameloblastic fibroma [2]. Its cystic counterpart, COC is more common. There has been ongoing debate around COC and its classification as a tumour or cyst [1]. When classified as a tumour, the COC accounts for 1-2% of odontogenic tumours [4]. The DGCT is much rarer and has always been recognised as a neoplasm [2]. There are reported cases of malignant transformation of DGCT [5]. The ghost cell odontogenic carcinoma can arise within an existing lesion or de novo and is differentiated from DGCT with p53 positivity and a high proliferative fraction [1]. There is a predilection for Asians and males (4:1 male-to-female ratio) [5]. The lesion presents within a wide age range, and occurs most commonly in the posterior maxilla and mandible. Peripheral (extraosseous) cases have been described [6]. As in this case, the most common presentation is an asymptomatic swelling with underlying cortical expansion.
Macroscopically the DGCT is solid with areas of calcification and microcystic spaces [1] (Figure 4, 5). Histologically, the lesion contains strands of ameloblastic-like odontogenic epithelium, aberrant keratin with associated calcifications, microcystic spaces and the presence of ghost cells [1]. The ghost cells are variable in number and can create diagnostic haze with ameloblastoma. However, their presence alongside dentinoid material is an important differentiation factor [1]. These cells are eosinophilic and lack nuclei, but maintain their cellular outline [5]. They can appear in a number of other lesions including odontoma, craniopharyngioma, ameloblastoma and ameloblastic fibroma [7]. Multinucleated giant cells can also be evident, due to a foreign body reaction that occurs when ghost cells extend beyond the basement membrane [1,8].
Enucleation or simple excision of DGCT has a reported recurrence rate of 73-100% [1,9]. Others recommend resection with a 5-millimetre margin, with a reported recurrence rate of up to 33% [1,5,9,10]. However, the literature is lacking; the largest published series identified includes only seven cases [9].
The case presented herein is unique for both its rare pathology and unique reconstructive design. Similar defects are often restored with soft tissue flaps, which provide suboptimal support for the upper lip, nasal aperture and peri-orbital facial contours, and make dental rehabilitation challenging. The fibula free flap has been suggested by others to be inadequate and overly complex for such defects [11]. We have demonstrated however that with appropriate planning, Brown’s class III defects can be suitably restored.
References
- El-Naggar AK, Chan JKC, Rubin Grandis J, Takata T, Slootweg PJ, International Agency for Research on Cancer. WHO Classification of Head and Neck Tumours. 1st ed. Lyon, France: International Agency for Research on Cancer, 2017.
- Tajima Y, Ohno J, Utsumi N. The dentinogenic ghost cell tumor. J Oral Pathol Med, 1986; 15(6): 359–362.
- Brown JS, Shaw RJ. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol [Internet]. Elsevier Ltd, 2010; 11(10): 1001–1008. http://dx.doi.org/10.1016/S1470-2045(10)70113-3
- Buchner A. The central (intraosseous) calcifying odontogenic cyst: An analysis of 215 cases. J Oral Maxillofac Surg, 1991; 49(4): 330–339.
- Biggs T, Hayes S, Harries P, Salib R. Maxillary dentinogenic ghost cell tumour. Ann R Coll Surg Engl, 2013; 95(3): 63–65.
- Wong YK, Chiu SC, Pang SW, Cheng JCF. Peripheral dentinogenic ghost cell tumour presenting as a gingival mass. Br J Oral Maxillofac Surg, 2004; 42(2): 173–175.
- Rajesh E, Jimson S, Masthan KMK, Balachander N. Ghost cell lesions. J Pharm Bioallied Sci [Internet]. 2015; 7(Suppl 1): S142–S144.
- Barpande S, Bhavthankar J, Singhaniya S. Dentinogenic ghost cell tumor. J Oral Maxillofac Pathol [Internet], 2009; 13(2): 97–100.
- Sun G, Huang X, Hu Q, Yang X, Tang E. The diagnosis and treatment of dentinogenic ghost cell tumor. Int J Oral Maxillofac Surg, 2009; 38(11): 1179–1183.
- Kasahara K, Iizuka T, Kobayashi I, Totsuka Y, Kohgo T. A recurrent case of odontogenic ghost cell tumour of the mandible. Int J Oral Maxillofac Surg, 2002; 31(6): 684–687.
- Rodriguez ED, Martin M, Bluebond-Langner R, Khalifeh M, Singh N, Manson PN. Microsurgical reconstruction of posttraumatic high-energy maxillary defects: Establishing the effectiveness of early reconstruction. Plast Reconstr Surg, 2007; 120(7 Supplement 2): 103–117.

