Aortitis and Atrioventricular Block following COVID-19 Infection
Yimeng Wang1,*, Nikola Wilk2, Anthony Ciarallo2, Fares Kalache3 and Thao Huynh4
1Resident Physician, Internal Medicine McGill University Health Centre, Canada
2Assistant Professor, Nuclear Medicine, McGill University Division of Diagnostic Radiology, Canada
3Assistant Professor, Medicine, McGill University Division of Rheumatology, Canada
4Associate Professor, Medicine, McGill University Division of Cardiology, Canada
Received Date: 02/04/2023; Published Date: 07/07/2023
*Corresponding author: Yimeng Wang, MD, Resident Physician, Internal Medicine McGill University Health Centre, Canada
Abstract
Introduction
Several types of cardiovascular (CV) complications have been reported with COVID-19. In this report, we describe the first known case of concomitant aortitis and atrioventricular block (AVB) following COVID-19 infection.
Case Presentation
A 40-year-old previously healthy African male presented with pericarditis, six weeks after a minimally symptomatic COVID-19 infection. He also developed transient AVB. He was started on high dose steroids and did not suffer any complications from his aortitis. The patient did not require any pacemaker insertion.
Conclusions
AVB and aortitis are two uncommon and distinct complications of COVID-19 infection. As these complications can occur in the subacute phase of a minimally symptomatic infection, it is of paramount importance that physicians remain vigilant beyond the acute infection, even in initially mild cases.
Keywords: Aortitis; Atrioventricular-block; AVB; COVID; B.1.1.7; UK-variant
Abbreviations: ACE2: Angiotensin Converting Enzyme2; AVB: Atrioventricular Block; CRP: C-Reactive Protein; CT: Computed Tomography; CV: Cardiovascular; FDG: Fluorodeoxyglucose; HR: Heart Rate; PET: Positron Emission Tomography
Background
Significant morbidity and mortality can arise from COVID-19 infection. COVID-19 infection has been associated with several types of cardiovascular (CV) complications including myocardial injury, myocarditis, acute myocardial infarction, heart failure and arrhythmia [1], which often manifest weeks following initial infection [2]. COVID-19 has also been associated rarely with aortitis and atrioventricular block (AVB) separately [3, 4]. To the best of our knowledge, we are the first to report a case of concurrent aortitis and AVB following a COVID-19 infection. In this report, we described the management and outcome of concurrent aortitis and AVB.
Case Presentation
Mr. C is a 40-year-old previously healthy African male who worked as a construction site supervisor. The patient acquired COVID-19 in March 2021 and had only minimal fatigue in the initial weeks of his infection.
Four weeks after his initial infection, Mr. C presented with pleuritic chest pain with diffuse ST- segment elevations on his electrocardiogram. Additionally, he also developed high grade AVB with a heart rate (HR) of 45 per minute.
Investigations
Laboratory investigation revealed an elevated C-reactive protein (CRP) at 98 milligrams/L. Tuberculosis QuantiFERON, cultures for histoplasma, Blastomyces, leishmania, HIV and Lyme serologies, four blood cultures, autoimmune screen and vasculitis work-up were all negative. High sensitivity troponin and thyroid function tests were normal. Transthoracic echocardiogram and a cardiac computed tomography (CT) were normal. A cardiac magnetic resonance imaging (MRI) showed acute left ventricular myocardial edema without fibrosis and a small circumferential pericardial effusion. Chest computed tomography was negative for active sarcoidosis.
Fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan (done for sarcoidosis work-up) showed a moderate degree of metabolic activity in the ascending aorta, suspicious for a vasculitis (Appendix 2). An initial syphilis enzyme immunoassay was positive, yet a rapid plasma regain test was negative. A confirmatory test was positive for endemic treponema syphilis.
Treatment
Due to the spontaneous improvement of HR to 70/min within a few days, the patient did not require a pacemaker. The patient was started on ibuprofen and colchicine for pericarditis. He was initiated on a tapering course of prednisone 40mg orally daily over four weeks for the aortitis. He also received intramuscular penicillin injections to treat the suspected endemic treponema.
Outcome Aand Follow-Up
At seven weeks after his initial COVID-19 infection, the patient still had mild intermittent chest pain without any other active CV, neurological or rheumatologic symptoms.
Appendix 1 – Initial electrocardiogram
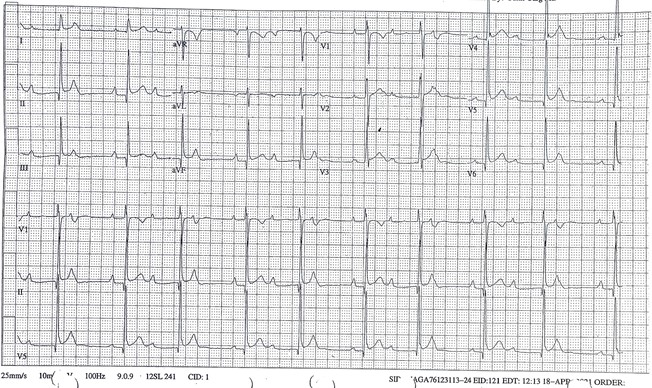
Figure 1: ECG dated April 28th, 2021 during hospital admission showing diffuse ST elevation, and ST depression in lead aVR typical of pericarditis as well as second degree AV block.
Appendix 2 - 18F-FDG PET-CT
Transaxial views of (A) fusion PET-CT image, (B) CT. Coronal views of (C) fusion PET-CT image, and (D) CT.
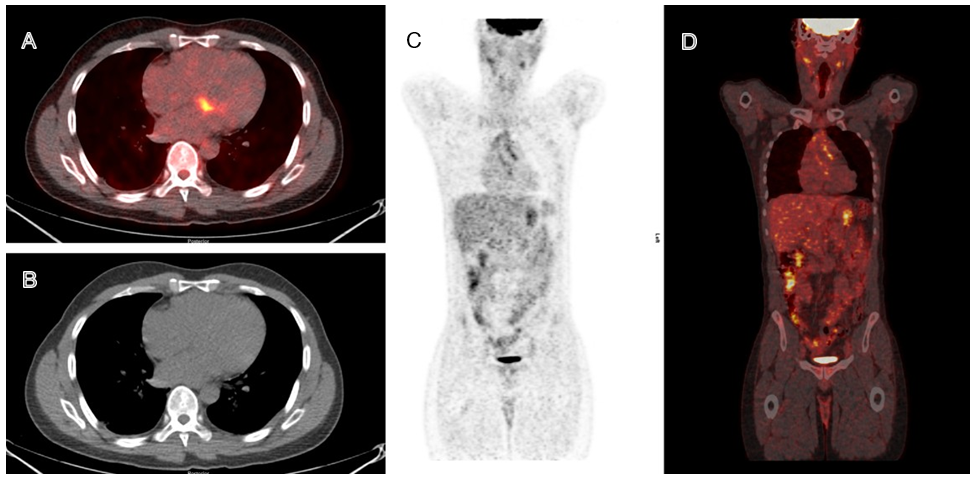
Figure 2: The presented images highlight the prominent focal hypermetabolism in the basal septum likely affecting the AV node in light of the clinical presentation of heart block. The metabolic activity is clearly delineated and localizes to the walls of the ascending aorta.
Discussion
To the best of our knowledge, this is the first reported case of aortitis complicated by AVB following a COVID-19 infection. The AVB was likely due to extension of the inflammation of the aorta to the AV node (adjacent to the aortic root).
Wang et al. reported a prevalence of 23% of cardiac arrhythmia in the initial Wuhan cohort of COVID-19 infected individuals [5]. The most frequent arrhythmia were atrial fibrillation, atrial flutter and ventricular tachycardia [6]. AVB were rare following COVID-19 infection [7-9]. These conduction abnormalities were thought to be due to acute inflammation of the intrinsic conduction system [7] and/or from direct viral injury to the myocardium [10]. AVB was generally transient (less than 1 week) and did not require a permanent pacemaker [6,7,11]. Temporary pacemakers were required in rare instances [12].
Kawasaki’s disease and cutaneous vasculitis following COVID-19 infection have been observed in children [13,14]. In adults, the vasculitis phenomena manifested mostly as thromboembolism, cutaneous vasculitis, and myopericarditis [15]. Four cases of inflammatory aortitis due to COVID.
19 have been reported [3, 16-18]. The spectrum of COVID-19 related aortitis symptoms varied widely from asymptomatic to fatigue, weight loss, chest, back and abdominal pain, claudication, neurological deficits, life-threatening aortic aneurysm and aortic dissection [3]. Acute COVID-19 aortitis is suspected to be due to an infiltration of virions into the aortic endothelium mediated by angiotensin-converting enzyme 2 (ACE2) receptors. The cellular viral invasion leads to acute endothelitis and leukocytoclastic vasculitis. This inflammatory cascade leads to deposition of immune complexes with a type-3 hypersensitivity reaction [17, 19-21]. Prior COVID-19 related aortitis have been successfully treated with two-to-four-week courses of 40-60mg of prednisone [3, 16].
FDG PET is recommended for the diagnosis of suspected aortitis, regardless of etiology. There are no current guidelines regarding PET use in the management of COVID-19 infection. Repeating the FDG PET three to six months following the infection may be helpful to evaluate the response to therapy. Additionally, FDG PET can also confirm relapse of large vessel vasculitis [22-24].
Although our patient tested positive for syphilis, it was unlikely that his aortitis was due to active venereal syphilis. He was from west Africa where the prevalence of endemic treponema was 33% [25]. It was most likely that he had endemic treponema infection, known as Bejel infection, which is not associated with any CV complication [25]. Furthermore, concomitant AVB and aortitis is exceedingly rare in venereal syphilis. Venereal syphilis is associated with gummas (granulomatous) infiltrating the conduction system [26]. The gummas would not have resolved spontaneously, as in our patient. Furthermore, this patient had no other finding suggestive of tertiary or CV syphilis.
The development of subacute CV complications following the initial COVID-19 infection emphasizes the need for health care providers to remain vigilant beyond the first few weeks of the infection. The median onset of CV symptoms following COVID-19 infection is 47 days [2]. Of note, our patient had only minimal symptoms with the initial COVID-19 infection. Therefore, mild COVID- 19 infection does not preclude late life-threatening complications.
Funding sources: None
Competing interests: The authors have no competing interests to disclose
Author contributions: YW and NW wrote the manuscript and performed a literature search; AC analysed the CT-PET imaging and provided background; FK provided the rheumatologic workup and background; TH proofread and edited the manuscript.
References
- Long B, et , Cardiovascular complications in COVID-19. Am J Emerg Med, 2020; 38(7): p. 1504-1507.
- Salerno M, Kwong RY. CMR in the Era of COVID-19. JACC: Cardiovascular Imaging, 2020; 13(11): 2340-2342.
- Dhakal P, et Aortitis in COVID-19. IDCases, 2021; 24: p. e01063.
- Sardana M, Scheinman MM, Moss JD. Atrioventricular block after COVID-19: What is the mechanism, site of block, and treatment? Heart Rhythm, 2021; 18(3): p. 489-
- Wang D, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, JAMA, 2020; 323(11): p. 1061-1069.
- Jean-Louis F, et al. A Rare Case of Resolution of High-Degree Atrioventricular Block Associated With COVID-19. J Med Cases, 2020; 11(8): 243-245.
- Dagher L, et High-degree atrioventricular block in COVID-19 hospitalized patients. Europace, 2021; 23(3): p. 451-455.
- Ashok V, Loke WI. Case report: high-grade atrioventricular block in suspected COVID-19 Eur Heart J Case Rep, 2020; 4(FI1): p. 1-6.
- Kochav SM, et al. Cardiac Arrhythmias in COVID-19 Infection. Circ Arrhythm Electrophysiol, 2020: 13(6): e008719.
- Madjid M, et al. Potential Effects of Coronaviruses on the Cardiovascular System: A JAMA Cardiol, 2020; 5(7): p. 831-840.
- Kir D, Mohan C, Sancassani R. Heart Brake: An Unusual Cardiac Manifestation of COVID-19. JACC Case Rep, 2020; 2(9): 1252-1255.
- Chinitz JS, et al. Bradyarrhythmias in patients with COVID-19: Marker of poor prognosis? Pacing Clin Electrophysiol, 2020; 43(10): 1199-1204.
- Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet, 2020; 395(10239): p. 1771-1778.
- SA Vasculitis in COVID-19: a literature review. J Vasc Surg, 2020; 1: p. 1-5.
- McGonagle D, et al. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol, 2021; 3(3): e224-e233.
- Zou Y, Bhavisha Vasta. COVID-19 associated aortitis. Rheumatology Advances in Practice, 2020;
- Manenti A, et al. Vasculitis and aortitis: COVID-19 challenging complications. J Vasc Surg, 2021; 73(1): 347-348.
- Shergill S, Davies J, Bloomfield J. Florid aortitis following SARS-CoV-2 Eur Heart J, 2020; 41(44): p. 4286.
- Pons S, et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 Crit Care, 2020; 24(1): p. 353.
- Roncati L, et Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol, 2020; 217: p. 108487.
- Jung F, et al. COVID-19 and the endothelium. Clin Hemorheol Microcirc, 2020; 75(1): p. 7-11.
- Hellmich B, et al. 2018 Update of the EULAR recommendations for the management of large vessel Ann Rheum Dis, 2020; 79(1): p. 19-30.
- Sollini M, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F] FDG-PET/CT Eur J Nucl Med Mol Imaging, 2021; 48(5): p. 1460-1466.
- Bruls S, et 18F-FDG PET/CT in the Management of Aortitis. Clin Nucl Med, 2016; 41(1): p. 28-33.
- Giacani L, Lukehart SA. The endemic treponematoses. Clinical microbiology reviews, 2014; 27(1): 89-115.
- Heggtveit Syphilitic aortitis: a clinicopathologic autopsy study of 100 cases, 1950 to 1960. Circulation, 1964; 29(3): p. 346-355.
- Song Y, GZ Cui S, Tian D, Wan G, Zhu S, Wang X, et al. COVID-19 cases from the first local outbreak of SARS-CoV-2 1.1. 7 variants in China presented more serious clinical features: a prospective, comparative cohort study, 2021.
- Davies NG, et al., Increased mortality in community-tested cases of SARS-CoV-2 lineage 1.1. 7. Nature, 2021; 593(7858): p. 270-274.

