Conversion of Hypothyroidism to Hyperthyroidism: Not So Rare a Phenomenon?
Pari Naz Qayum1,*, May Thin Khine2 and Parijat De3
1Specialty Registrar Sandwell and West Birmingham NHS Trust, United Kingdom
2Endocrine Registrar Sandwell and West Birmingham NHS Trust, United Kingdom
3Consultant Physician (Diabetes & Endocrinology) Sandwell and West Birmingham NHS Trust, United Kingdom
Received Date: 20/03/2023; Published Date: 26/06/2023
*Corresponding author: Pari Naz Qayum, MBBS, MRCP, Specialty Registrar Sandwell and West Birmingham NHS Trust, United Kingdom
Abstract
It is generally thought that over-replacement with thyroxine hormone is the main reason for the biochemical transformation to hyperthyroidism in patients with autoimmune hypothyroidism. However, it might not be the only cause - a rare form of autoimmune entity might be the contributing factor as well involving switching between TSH receptor Stimulating Antibodies (TSAb) and TSH receptor Blocking Antibodies (TBAb). We present a case of spontaneously oscillating thyroid function over a 15‐year period in a 55-year-old lady who was initially diagnosed with thyrotoxicosis in 2006 for which she had anti-thyroid medications for 18 months. Subsequently, she developed hypothyroidism and was started and maintained on L-thyroxine replacement for many years. She then developed hyperthyroidism (on previously stable dose of thyroxine replacement) in 2019 which was confirmed by the presence of TSH-receptor antibody, needing anti-thyroid treatment for 2 years.
Keywords: Autoimmune hypothyroidism; TSH receptor stimulating antibodies (TSAb); TSH receptor blocking antibodies (TBAb); Antithyroid treatment
Introduction
Although cases of conversion from hyperthyroidism to hypothyroidism are often encountered in clinical practice, it is rare to see conversion of primary hypothyroidism due to Hashimoto’s disease to hyperthyroidism [1,2]. The exact incidence of this conversion is not known due to its presumed rarity -it is possible that such conversion may be due to development of blocking and stimulating antibodies. Treatment is straightforward with anti-thyroid medications once the diagnosis is suspected and confirmed. Therefore, it is important to get the correct diagnosis to be able to treat such patients appropriately.
Case Report
A 55-year-old lady was referred to us by her GP in 2013 with symptoms of hypothyroidism - tiredness, shortness of breath on exertion, weight gain, hair loss and brittle nails and skin. She was initially diagnosed with Grave’s thyrotoxicosis in 2006 for which she had anti-thyroid medication for 18 months following which she became hypothyroid needing treatment with levothyroxine which was started in January 2010. She continued to remain symptomatic with tiredness, weight gain, joint pains and mental slowing despite being on levothyroxine and maintaining a normal TSH level varying between 1.46mU/L – 2.00mU/L (0.5-5.0mU/L). She was therefore started on a combination of Liothyronine 20,20,10mcg three times daily and Levothyroxine 25mcg alternate days which was started in 2010.
Her past history included pre-diabetes and she was taking oral Vitamin D and B12. She worked as a sales manager. She was a non-smoker and a teetotaler with no family history of thyroid disease.
She had been euthyroid from 2014 till 2019. In April of 2019, she stopped her thyroxine treatment. However, as she started to experience symptoms of hyperthyroidism in May 2019 including palpitations, tremors, anxiety, sleep disturbances, brittle nails and hair loss. The diagnosis of hyperthyroidism was confirmed with a TSH < 0.01mU/L (0.5-5.0mU/L), raised FT3 of 18.8pm/L (2.0-7.0) and a FT4 of 29.3pm/L (9.0-25.0). At this stage, both her TPO and TRAB antibody levels were positive.
Stopping her treatment resulted in slight improvement in her biochemical profile with FT3 of 13pm/L (2.0-7.0), FT4 of 28pm/L (9.0-25.0) and TSH < 0.01mU/L (0.5-5.0mU/L) but she continued to remain symptomatic. Bloods showed raised anti-TPO antibody of 540kIU/L (0.0-34.0). She was therefore started on antithyroid medication propylthiouracil (PTU) 100mg twice daily in August 2019 which led to significant improvement in her symptoms and TFTs. Her PTU dose was reduced to 50 mg twice daily in December 2019 and this was then stopped in June 2020. The patient remained euthyroid subsequently following which she was discharged back to her GP.
Just before the onset of her hyperthyroidism symptoms, she had developed symptoms of menopause (hot flushes and insomnia) and was being treated with topical estrogel. She was also diagnosed with pre-diabetes for which she was started on metformin. It is difficult to ascertain what exactly caused this conversion, which happened to coincide with her menopausal symptoms.
Investigations
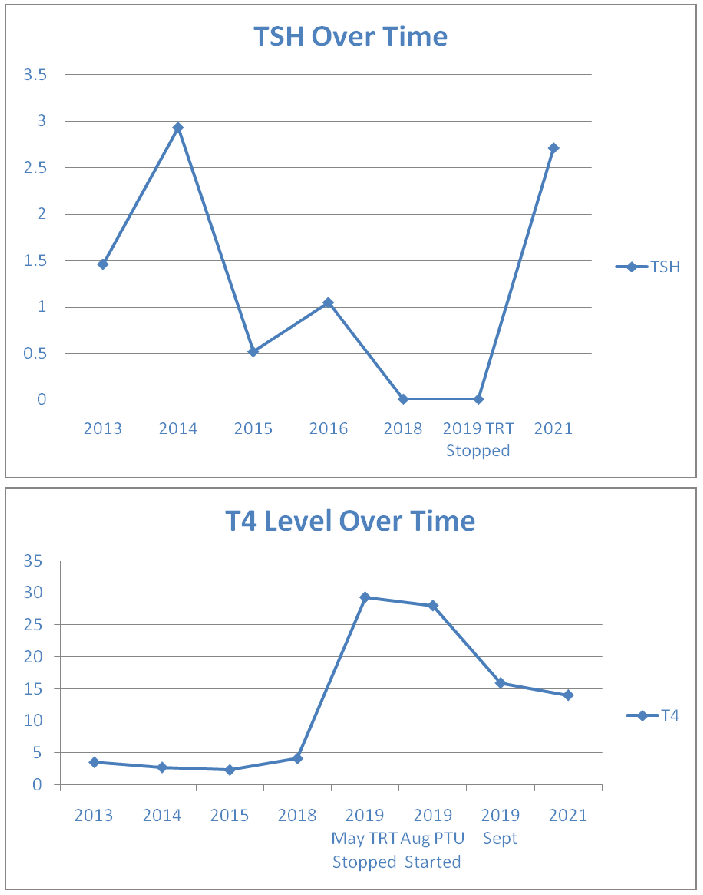
Her blood results showed a stable euthyroid picture on thyroxine replacement until 2019, when she developed a hyperthyroid picture (Figs 1 and 2). The anti-TPO antibody levels were high at 540U/mL in May 2019 reducing somewhat to 235.0 kIU/L in Jan 2020. TRAB was also elevated at 15.8 IU/L (<1.8 IU/L) confirming the autoimmune basis. No TSAb was checked. Only diagnostic, and not serial, TRAB was performed as per local policy. A full autoimmune screen was not performed, coeliac screen was negative.
Treatment/Outcomes
To summarise, our patient with stable hypothyroidism developed clinical symptoms of thyrotoxicosis with biochemical thyrotoxicosis and positive TRAb, all in keeping with a diagnosis of autoimmune hyperthyroidism needing anti-thyroid medications for nearly 1 year (from August 2019 to June 2020) with subsequent complete clinical and biochemical resolution.
Discussion
In this case report, we describe a middle-aged lady who was initially diagnosed with thyrotoxicosis in 2006 following which she became hypothyroid after 18 months of anti-thyroid treatment. She remained euthyroid on stable doses of levothyroxine for 11 years before becoming hyperthyroid with strongly positive TRAB antibody and TPO antibody levels.
The first similar case was described by Joplin and Fraser in 1959 [3] and was followed by several others in the later 60s and 70s [4-6]. In 1990 Takasu et al. [2] described a case series of eight cases converting to Graves’ disease following Hashimoto’s disease, and it was observed that those cases could be divided into three groups: group of transient Graves’ disease following hypothyroidism, group of persistent Graves’ thyrotoxicosis following hypothyroidism and group of persistent hypothyroidism despite positive thyroid stimulating immunoglobulins.
What causes this conversion is not well understood, but there are different theories postulated. In this case, it happened at the same time when patient went through menopause and whether this could implicate a trigger in a genetically susceptible individual is a possible mechanism.
One early study [5] as well as that of Takasu et al. [2], suggests that the alterations in thyroid state were related to the balance in the activity of stimulating and blocking antibodies and the response of thyroid gland to these antibodies. More recently, Moriarty et al. [6] described a case of conversion of autoimmune hypothyroidism to hyperthyroidism with thyroid eye disease. They believe that variable behaviour of TRAB with the TSH receptor is responsible for the conversion from hypothyroidism to hyperthyroidism and vice versa.
Furgan et al. [1], who also described three cases of autoimmune hypothyroidism converting to hyperthyroidism, proposed two theories to explain this conversion: first, is the presence of both blocking and stimulating antibodies causing a pull–push effect shifting either to hypothyroidism or hyperthyroidism respectively, and a second theory, that thyroid damage from an autoimmune phenomenon initially causes thyroid hypo-function but once enough tissue has recovered, it is stimulated by stimulating antibodies causing thyroid hyper-function. They also suggested that this conversion of hypothyroidism to hyperthyroidism may not be as rare as previously thought.
One of the largest recent studies was done by Takasu and Matsushita [7]. They identified 34 patients with hypothyroidism with TSH receptor blocking antibodies and 98 patients with Graves’ disease with TSH receptor-stimulating antibodies and followed the natural course over 10 years. They concluded that hypothyroidism due to blocking antibodies and hyperthyroidism with stimulating antibodies may be two spectrums of the same condition or disease. It is also postulated that genetically susceptible individuals may require an external trigger to switch from autoimmune hypothyroidism to Graves’ disease, such as an infection or neck irradiation [9,10], which would tilt the balance to shift from TSH receptor-blocking antibodies to TSH receptor-stimulating antibodies.
In conclusion, this phenomenon of autoimmune conversion, may not be as rare as previously thought. Physicians should be aware of this possibility especially if someone with a previously well-established hypothyroidism starts to require less or no thyroid hormone replacement, or even develops overactive thyroid symptoms with biochemical hyperthyroidism for no obvious reason (even many years after the diagnosis of hypothyroidism, as in this case). Further research, however, is needed to establish the exact mechanism of this phenomenon as the exact pathophysiological trigger and aetiology is still not fully understood.
Author contribution statement: Dr Parijat De was the lead clinician managing the patient’s thyroid disorder; Dr Pari Naz Qayum was the clinician trainee who wrote the initial main draft; Dr May Thin Khine reviewed the draft case report and amended changes as needed.
Competing interests: None
Patient consent: Written informed consent was obtained by Dr Pari Naz Qayum.
References
- Furgan S, Haque NU, Islam N. Conversion of autoimmune hypothyroidism to hyperthyroidism. BMC Research Notes, 2014; 7: https://doi.org/10.1186/1756-0500-7-489.
- Takasu N, Yamada T, Sato A, Nakagawa M, Komiya I, Nagasawa Y, et al. Graves’ disease following hypothyroidism due to Hashimoto’s disease: studies of eight cases. Clinical Endocrinology, 1990; 33: 687–698. https://doi.org/10.1111/j.1365-2265.1990.tb03906.x
- Joplin GF, Fraser R. Thyrotoxicosis developing in recurrent nodular goitre with focal thyroiditis. Proceedings of the Royal Society of Medicine, 1959; 52: 177–178.
- Doniach D, Hudson RV, Roitt LM. Human autoimmune thyroiditis: clinical studies. BMJ, 1960; 1: 365–373. https://doi.org/10.1136/ bmj.1.5170.365.
- Goolden AWG, Davidson M, Hoffenberg R. Myxedema preceding hyperthyroidism. Lancet, 1971; 2: https://doi.org/10.1016/S0140- 6736(71)92611-0.
- Irvine WJ, Lamberg BA, Cullen D, Raud-Gordin R. Primary hypothyroidism preceding thyrotoxicosis. Journal of Clinical and Laboratory Immunology, 1979; 8: 3–19.
- Moriarty M, Mills E, Yap HL, Hamda A. Conversion of autoimmune hypothyroidism to hyperthyroidism with thyroid eye disease. Endocrine Abstracts, 2015; 37: https://doi.org/10.1530/ endoabs.37.EP1307.
- Takasu N, Matsushita M. Changes of TSH-stimulation blocking antibody (TSBAb) and thyroid stimulating antibody (TSAb) over 10 years in 34 TSBAb-positive patients with hypothyroidism and in 98 TSAb-positive Graves’ patients with hyperthyroidism: reevaluation of TSBAb and TSAb in TSH-receptor-antibody (TRAb)-positive patients. Journal of Thyroid Research, 2012; 2012: https://doi. org/10.1155/2012/182176.
- Lewandowski K, Dąbrowska K, Makarewicz J, Lewiński A. Pendulum swings from hypo- to hyperthyroidism: thyrotoxicosis after severe hypothyroidism following neck irradiation in a patient with a history of Hodgkin’s lymphoma. Thyroid Research 2016; 9: https:// doi.org/10.1186/s13044-016-0030-1.
- Al-Sharafi BA, Khardori R. Hyperthyroidism after hypothyroidism. South Med J, 2000; 93: 703–707.

