Tracheal Replacement with Cryopreserved Aortic Homograft Maintained by Custom 3D Printed Plastic External Splint
Mariano Boglione*, Lucía Gutiérrez Gammino, Carlos Giuseppucci, Ignacio Berra, Ramiro Ortíz, Laura Galluzzo, José Lipsich, Aixa Reusmann, Martín Cadario and Marcelo Barrenechea
Departments of Surgery, Pathology and Radiology, Hospital de Pediatría Prof. Juan P. Garrahan, Argentina
Received Date: 27/03/2023; Published Date: 22/06/2023
*Corresponding author: Mariano Boglione, MD, Departments of Surgery, Pathology and Radiology, Hospital de Pediatría Prof. Juan P. Garrahan, Buenos Aires, Argentina
Abstract
Many techniques have been described to solve tracheal stenosis including replacement with cryopreserved aorta. The use of “splints” as external support of the airway in cases of tracheo and bronchomalacia has also been reported. Herein we report an experimental case of tracheal replacement in a rabbit using a cryopreserved aortic graft supported externally by a plastic 3D printed custom-made exoskeleton (“splint”).
Five rings of cervical trachea were replaced in a 2,5 kg New Zealand white rabbit. The animal was sacrificed 60 days after implantation. During this time, it showed adequate respiratory parameters to carry out their daily activities of walking and feeding. Histologic study observed normal lung structure; and loss of elastic fibers and polymorphonuclear cells infiltrate in the aorta wall.
We believe that the use of cryopreserved aortic segments supported externally by an exoskeleton (“splint”) offers a valid alternative in tracheal replacement surgery.
Keywords: Tracheal estenosis; Cryopreserved aorta; Splint
Introduction
Tracheal stenosis symptoms vary depending on the patient's age, the severity of the stenosis and the presence of associated malformations [1].
Its management represents a surgical challenge because the stenosis may have different lengths, appear at different levels of the trachea and can be associated with pathologies that also need surgical treatment in the same act.
Tracheal reconstruction is necessary in cases of acquired stenosis (secondary to prolonged intubation, tracheostomy, trauma or neoplasia) that does not respond to medical treatment with periodic dilations and also in cases of congenital stenosis characterized by the presence of a variable number of complete cartilaginous rings generating a fixed narrowing of the trachea that makes its dilation impossible [2,3].
All described techniques have complications (stenosis, dehiscence, collapse) that in some cases can be fatal. Years ago, our group placed a cryopreserved aortic graft in a 4-year-old girl as a salvage procedure in a severe complication after laryngotracheal reconstruction [4]. Lacking its own support structure, the cryopreserved aorta collapses with inspiratory movements, so it is necessary to maintain its lumen using a tracheostomy cannula or a permanent dilator (“stent”).
Recently, Martinod and colleagues have used autogenous aortic grafts [5,6] and allogeneic aortic grafts [7] to replace long segments of tracheal and carina [8] defects using sheep as an animal model with promising results. Seguin and colleagues have used a decellularized, cryopreserved aortic allograft supported by a temporary stent to prevent airway collapse [9].
The use of “splints” as external support of the airway in cases of tracheo and bronchomalacia [10,11] has also been reported.
An exoskeleton-expanded aortic graft could be used as airway segment replacement without the need for stents or tracheostomy tubes [12,13].
The purpose of this experience is to evaluate the feasibility of replacing a segment of the trachea with a cryopreserved aortic graft supported externally by a plastic 3D printed custom-made exoskeleton (“splint”) based on the tomographic image of the trachea [14,15].
Case Report
Animal
A New Zealand white rabbit, outbred strain, weighing 2.5 kg, was used. A segment of 5 tracheal rings was replaced by an equal length segment of cryopreserved aorta supported externally by a plastic exoskeleton.
The animal received humane treatment in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council. Eighth Edition. National Academies Press, Washington DC, 2011 and/or European Union Directive 2010).
Cryopreservation
The descending thoracic aorta was obtained from New Zealand rabbits weighing 2.5 kg.
Once dissected, the aorta was submerged in an incubation solution made up of saline solution with piperacillin, gentamicin, colistin, cefuroxime, and amphotericin B; and placed in a refrigerator at 4ºC for 7 days. Dimethylsulfoxide (DMSO), albumin and glycerol (preservation solution) were then added, and the temperature was gradually lowered to -80º C.
Before being implanted, the grafts were warmed by placing the container in a water bath at 37ºC for 10 minutes.
Making of the splint (exoskeleton)
A cervicothoracic CT scan of the rabbit was carried out to obtain images of the rabbit's airway. Processing DICOM´s CT scan, an exoskeleton was made in plastic material according to the size of the trachea using a 3D printer.
Implant
Anesthetic induction was performed with ketamine 40 mg/kg + midazolam 0.2 mg/kg + intramuscular atropine 1 mg/kg. Once sedation was achieved, the animal was placed on the table, a peripheral line was secured in the marginal vein of the ear, and propofol 2.2 mg/kg was administered to connect it to a mechanical ventilation system with a mask. Anesthetic maintenance was carried out with 2% isoflurane, fentanyl 2 gamma/kg/hour, and atropine every 30 minutes.
At the time prior to implantation, the cryopreserved aorta was placed within the exoskeleton and kept expanded by using non-absorbable sutures (Prolene®) from the exoskeleton to the aorta (Figure 1).
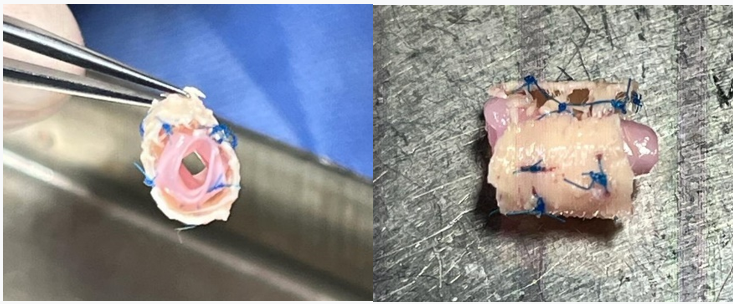
Figure 1: Cryopreserved aorta placed within the exoskeleton and kept expanded by using non-absorbable sutures (Prolene®) from the exoskeleton to the aorta.
A median cervicotomy was performed, with divulsion of the pretracheal muscles and dissection of the trachea. A 5-ring segment was sectioned and resected. It was then replaced by an equal length tube of cryopreserved aorta supported externally by a plastic splint (exoskeleton). The aorta was sutured to both tracheal ends using a continuous 5/0 nylon suture (Prolene®) (Figures 2 and 3). The muscle planes and skin were sutured with 4/0 and 5/0 absorbable polyglactin (Vicryl®) suture.
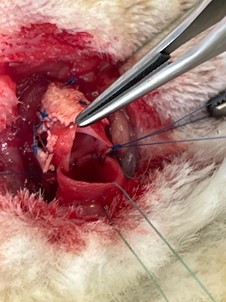
Figure 2: Posterior wall running suture among trachea and aorta.
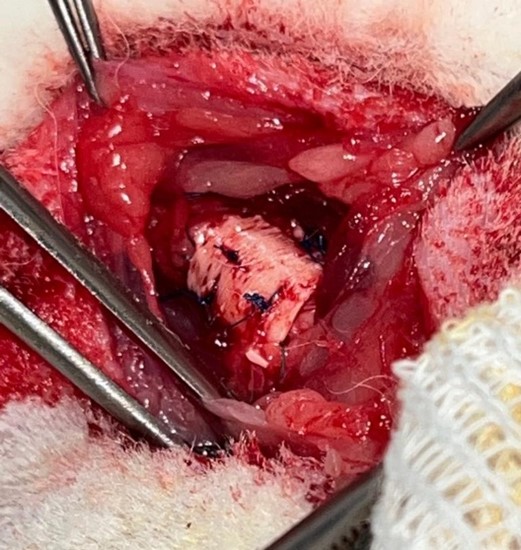
Figure 3: Splint grafted into the trachea.
Variables analyzed
Once the graft was placed, rabbit's breathing, development of daily activities (walking, feeding), and survival were evaluated by clinical examination, observation, and auscultation.
The animal was sacrificed 60 days after implantation, and the implanted specimen was analyzed macroscopically and microscopically (hematoxylin-eosin staining, PAS, Masson's trichrome).
Results
The rabbit survived the surgical procedure, and after anesthetic recovery showed adequate respiratory parameters to carry out their daily activities of walking and feeding.
Auscultation registered good air entry into both lungs with some rhonchi coming from the cervical trachea.
At sacrifice, granulomatous tissue was observed around the plastic exoskeleton.
The histopathological study reported:
Larynx: Normal structure.
Trachea: Normal structure. Slight inflammatory infiltrate in areas adjacent to the suture.
Lung: Normal structure (no edema or signs of infection observed) (Figure 4).
Aorta (graft): Loss of elastic fibers. Invasion of polymorphonuclear cells (abscessed areas in the center of the wall). Among these areas, presence of lymphoplasmacytic infiltrate and formation of neovessels (Figure 5). Absence of respiratory epithelium (Figure 6).
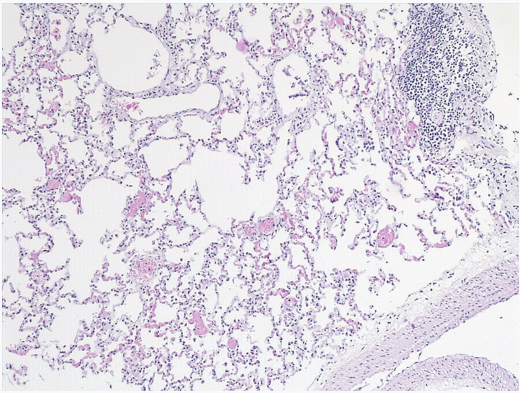
Figure 4: Lung: Normal structure.
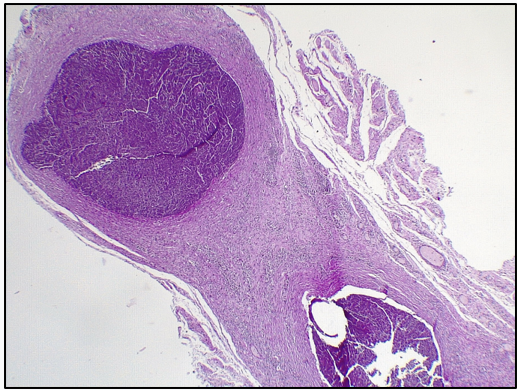
Figure 5: Aorta: Invasion of polymorphonuclear cells (abscessed areas in the middle of the wall). Among these areas, presence of lymphoplasmacytic infiltrate and formation of neovessels. Absence of respiratory epithelium.
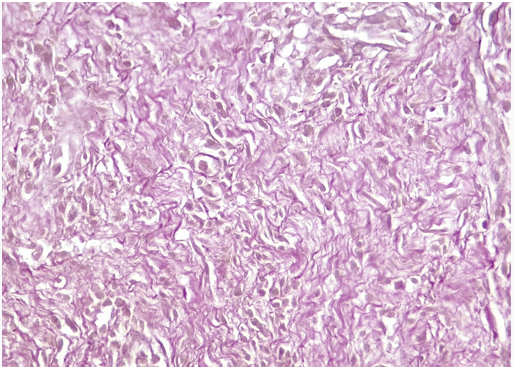
Figure 6: Aorta: Loss (absence) of elastic fibers.
Discussion
In the case presented, the cryopreserved aortic graft supported externally by an exoskeleton (“splint”), allowed the animal to breathe correctly and be able to feed and walk.
Martinod, in his study in twenty-two sheep to which he implanted a segment of fresh (recently obtained) aorta, used "stents" to prevent airway collapse [16]. In other study, Seguin also used stents to maintain the airway lumen when he replaced trachea segments with cryopreserved, decellularized, or glutaraldehyde-treated aorta grafts [18]. This author, in a study carried out on 15 sheep in which he replaced the tracheobronchial bifurcation with an aortic graft, pointed out the need to place stents to prevent their collapse [17]. Unlike these authors, in our case it was not necessary to use "stents", since the exoskeleton ("splint") was able to maintain an adequate diameter of the lumen of the implanted aortic graft.
Histopathological study showed no in-growth of respiratory epithelium into aorta´s wall; probably, this may vary in studies with longer survival.
Although the splint we used was made of plastic (a non-resorbable material), reabsorbable splints could be made, such as those reported by Cuestas [19] and Bellía-Munzón [20].
We believe that the use of cryopreserved aortic segments supported externally by an exoskeleton (“splint”) offers a valid alternative in tracheal replacement surgery.
Authors Contribution: The authors would like to thank the team of technicians and veterinarians Natalia Lausada, Gustavo Williams and Marcelo Asprea for their outstanding collaboration.
References
- DeLorimier A, Harrison M, Hardy K, Howell L, Adzick N. Tracheobronchial obstruction in infants and children: experience with 45 cases. Ann Surg, 1990; 212: 277-289.
- Cantrell JR, Guild H: Congenital stenosis of the trachea. Am J Surg, 1964; 108: 297-305.
- Chung SR, Yang JH, Jun TG, Kim WS, Kim YH, Kang IS, et al. Clinical outcomes of slide tracheoplasty in congenital tracheal stenosis. Eur J Cardiothorac Surg, 2015; 47(3): 537-542.
- Zanetta A, Cuestas G, Rodríguez H, Tramonti N, Boglione M. Reconstrucción laringotraqueal con aloinjerto de aorta criopreservada como rescate de una complicación de resección cricotraqueal en pediatría. Acta Otorrinolaringológica, 2012. http//dx.doi.org/10.1016/j.otorri.2012.07.006.
- Martinod E, Zegdi R, Zakine G, Aupecle B, Fornes P, D'audiffret A. A novel approach to tracheal replacement: the use of an aortic graft. J Thorac Cardiovasc Surg, 2001; 122:197-198.
- Martinod E, Seguin A, Pfeuty K, Fones P, Kambouchner M, Azorine JF, et al. Long-term evaluation of the replacement of the trachea with an autologous aortic graft. Ann Thorac Surg, 2003; 75: 1572-1578.
- Martinod E, Seguin A, Holder-Espinasse M, Kambouchner M, Duterque-Coquillaud M, Azorine JF, et al. Tracheal regeneration following tracheal replacement with an allogenic aorta. Ann Thorac Surg, 2005; 79: 942-949.
- Seguin A, Martinod E, Kambouchner M, Campo G, Dhote P, Bruneval P, et al. Carinal replacement with aortic allograft. Ann Thorac Surg, 2006; 81: 1068-1075.
- Seguin A, Radu D, Holder-Espinasse M, Bruneval P, Fialaire-legendre A, Duterque-Coquillaud M, et al. Tracheal replacement with cryopreserved, decellularized, or glutaral-dehyde-Treated Aortic Allografts. Ann Thorac Surg, 2009; 87: 861-867.
- Cuestas G, Doormann F, Bellia Munzon P, Bellia Munzon G. Biodegradable airway stent for the treatment of bronchial obstruction in the child. Case report. Arch Argent Pediatr, 2018; 116(1): 125–129.
- Bellia-Munzón G, Cieri P, Toselli L, Cuestas G, Doormann F, Gabaldón-Massé P, et al. Resorbable airway splint, stents, and 3D reconstruction and printing of the airway in tracheobronchomalacia. Sem Pediatr Surg, 2021; 30. https://doi.org/10.1016/j.sempedsurg.2021.151063

