Acute and Early Cardiotoxicity in a Patient with Hodgkin's Disease treated with the BEACOPP Protocol
Sayarh Salma*, Fellat Rokya and Fellat Nadia
Service of medical réanimation and cardiology A of the CHU Ibn Sina Rabat / University Mohammed V of Morocco, Morocco
Received Date: 23/03/2023; Published Date: 19/06/2023
*Corresponding author: Sayarh Salma, Department of Vascular Surgery, Ibn Sina Rabat University Hospital, Mohamed V University of Rabat, Morocco
Abstract
As part of the growth of Hodgkin lymphoma, several drugs are often combined. Combination chemotherapy reduces the risk of drug resistance. This combination is called protocol.
The reference protocols used in the treatment of Hodgkin lymphoma are the ABVD and BEACOPP protocols. The BEACOPP protocol is generally used as an “escalated” BEACOPP protocol, which means that the doses of are increased. The BEACOPP regimen (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) was initially developed to improve the first-line treatment of advanced Hodgkin Lymphoma (HL); however, its toxicity is polymorphic, it is dose-dependent and different organs can be affected, including the heart.
Cardiovascular (CV) toxicity induced by hemato-oncology treatments is a growing clinical problem [1,2]. Combination therapy is therefore difficult and requires a multidisciplinary approach involving cardiologists and specialists in hematology-oncology to minimize cardiotoxic effects.
We report the observation of cardiotoxicity in a man treated for Hodgkin lymphoma, in whom the etiological investigation revealed acute cardiotoxicity due to the BEACOPP protocol.
The publication of the ESC 2022 cardio-oncology recommendations is an important step in the structuring of this specialty: cardio-oncology.
The definition of cardiac dysfunction secondary to anti-cancer treatments was revisited in the 2022 guidelines. It should be classified according to whether it is symptomatic or not, and the degree of LV systolic dysfunction.
Introduction
During the past few decades Hodgkin’s Disease (HD) has become one of the most curable neoplasms. The survival rate of patients at each stage of HD has improved dramatically. Successful treatment for HD is 85-90% effective in the early stages and approximately 70-80% effective in advanced stages [1,2]. The treatment depends strictly on the disease stage and involves radiotherapy and chemotherapy and the recently introduced high-dose chemotherapy with peripheral stem cell transplantation for relapsed disease. In the early stage of HD an excellent survival rate of approximately 90% is achieved with the use of standard treatment protocols. Because of the high relapse rate after the first-line treatment of advanced HD, the standard regimens were modified and new protocols were introduced in the treatment of advanced HD. The introduction of regimen BEACOPP has significantly improved the prognosis for patients with advanced HD (2). However, his toxicity is polymorphic, and dose dependent, different organs can be affected.
Case Report
We report the case of a 38-year-old patient having as cardiovascular risk factors chronic smoking at a rate of 07 pack years, not known hypertensive or diabetic, followed for Hodgkin lymphoma for 02 months placed under BEACOPP chemotherapy protocol at a rate of a cure every 21 days (Cyclophosphamide 1250mg / m2 IV D1; Adriamycin 35mg / m2 IV D1; Vincristine 1.4mg / m2 IV D8; Bleomycin 10mg / m2 IV D8, Ectoposide 200mg / m2 IV D1 to D3; Procarbazine 100mg / m2 PO D1 to D7) with a strictly normal pre- chemotherapy assessment: clinical examination without particularities, biological assessment with a correct level of BNP and Pro BNP and an ETT estimating a LVEF at 55%, admitted to the emergency room on D8 of his 3rd treatment of chemotherapy in a table of global predominantly left heart failure motivating his hospitalization, and the performance of a transthoracic echocardiography showing a considerable drop in his LVEF to 27%.
In the process and in order to verify how LH responds to treatment after two cycles of chemotherapy as described in the literature, a thoracic-abdominal-pelvic scanner was performed objectifying intra and retro peritoneal as well as bilateral pelvic polyadenopathies associated with nodular splenomegaly and bone lesions evoking secondary locations, a positron emission tomography (P.E.Tscan ) was also done describing hypermetabolic foci above and below diaphragm: ganglion, hepatic and bone.
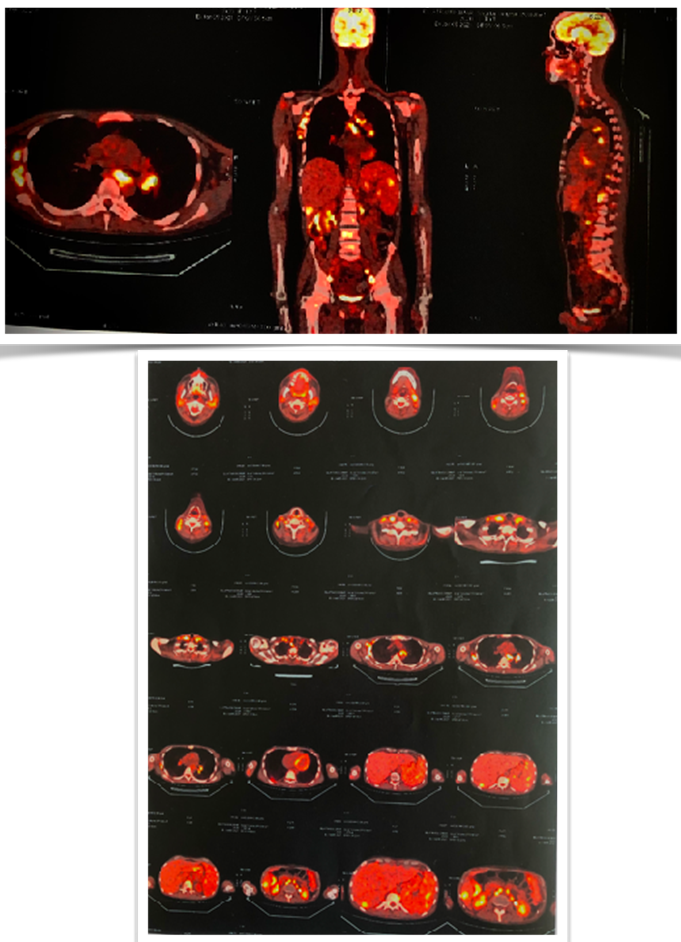
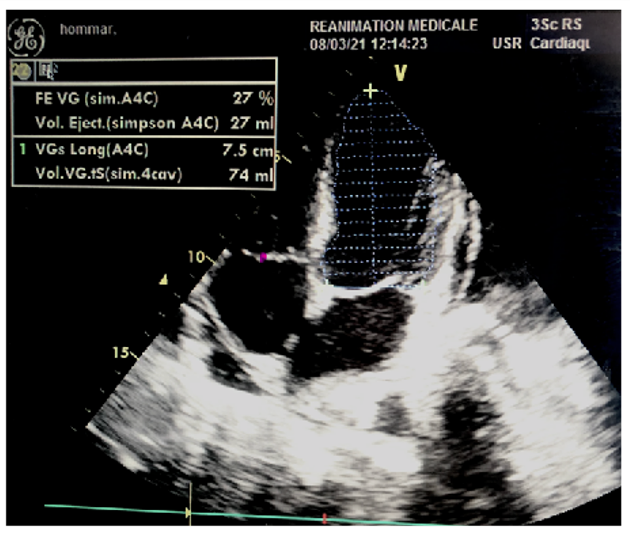
Discussion
Cardiotoxicity is defined as any CV event secondary to hematology-oncology treatment. Heart failure has traditionally attracted the most attention, but with the development of targeted therapies and longer survival times among patients with cancer, vascular toxicity is becoming more common [3].
CV disease prevention measures are important in patients with cancer and poor adherence is associated with an increased incidence of CV events [4].
To ensure coordinated care provision, both hematology-oncology specialists and patients need to be aware of the importance of monitoring CV risk.
Cardiovascular complications secondary to oncological management are numerous (systolic dysfunction of the left ventricle, acute myocarditis, arterial hypertension, prolongation of the QT interval, arterial or venous thrombosis), of variable frequency and mechanisms depending on the chemotherapy administered.
Patients with Hodgkin's disease, a form of cancer of the lymphatic system, are often treated with combination chemotherapy. This is then referred to as "multidrug therapy". In the case of advanced stage Hodgkin, the very effective reference protocol consists of a series of 8 courses of BEACOPP, a combination of seven drugs (Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, Prednisone) at high doses (or "escalated BEACOPP"). Some of these molecules, however, have problematic side effects.
The nature and frequency of cardiac complications encountered depend on the antimitotic agent administered, its cumulative dosage, and also on the combinations in which it is included (potentiation).t Bleomycin essentially describes pulmonary toxicity (pulmonary fibrosis, interstitial PNP), no hematological or cardiac toxicity, or alopecia. The occurrence of hypotension has not been correlated with cardiac toxicity or electrocardiographic changes. To prevent this rare adverse effect, it is recommended that etoposide be administered as a slow intravenous infusion over a period of 30 to 60 minutes. If arterial hypotension occurs, it usually responds to supportive therapy after discontinuation of treatment. Upon resumption of infusion, it is recommended that a slower infusion time be chosen. Other toxicities have been described with etoposide including hematologic toxicity, gastrointestinal toxicity, alopecia as well as allergic reactions and secondary leukemia. Treatment may cause a transient decrease in white blood cell count, which is maximal between days 7 and 14 after infusion and spontaneously reversible by day 21. This decrease increases the risk of infection, so consult your doctor promptly if you develop a fever. Among the alkylating agents, Cyclophosphamide can lead to severe cardiac damage [5], which may be manifested only by asymptomatic electrocardiographic changes such as microvoltage, QT interval prolongation or T wave and ST segment abnormalities. However, rhythm or conduction disorders, episodes of cardiac decompensation or even true cardiogenic shock may occur [6].
Cyclophosphamide also causes myocardial ischemia due to vasospastic and thrombotic phenomena at the vascular level. This cardiotoxicity is not correlated to the cumulative dose but rather to the size of a dose. All these complications are more frequent in the elderly, in patients who have already been treated with anthracyclines or who have undergone mediastinal irradiation [7], and may also be responsible for toxicity to the sexual glands, with the risk of more or less definitive cessation of menstruation in women and reduced fertility in men (preventive sperm freezing may be proposed). Hair loss is possible, but is always reversible after treatment has been stopped.
Doxorubicin, or, also known by the trade name Adriamycin, belongs to the family of anthracyclines. Anthracyclines (ATCs) are an essential therapeutic option in the management of many patients with cancer (breast cancer, Hodgkin's or non-Hodgkin's lymphoma, sarcoma), significantly improving their prognosis. However, this efficacy comes at the cost of cardiac toxicity ranging from asymptomatic LV dysfunction to heart failure, with one of the poorest prognoses of non-ischemic dilated heart disease.
The ESC 2022 recommendations devote a great deal of attention to the management of patients treated with TCAs, including risk assessment and stratification prior to prescribing TCAs, describing management procedures in the event of proven cardiac toxicity, and monitoring patients after TCA administration.
The definition of cardiac dysfunction secondary to treatment should be classified according to whether it is symptomatic or not and the degree of LV systolic dysfunction (Table 1).
Table1: Definition of myocardial cardiotoxicity to cancer treatment.

Risk assessment for anthracycline cardiotoxicity
The ESC 2022 guidelines emphasize the importance of evaluating all patients with cancer who are to receive cardiotoxic therapy to assess their risk of cardiotoxicity (Class I).
The HFA-ICOS risk score is used (Table 2), which is based on the patient's cardiovascular history, cardiac biomarker parameters, TTE and oncological history.
Table 2: stratification of the risk of cardiac toxicity secondary to the administration of anthracyclines according to the HFA-ICOS score.
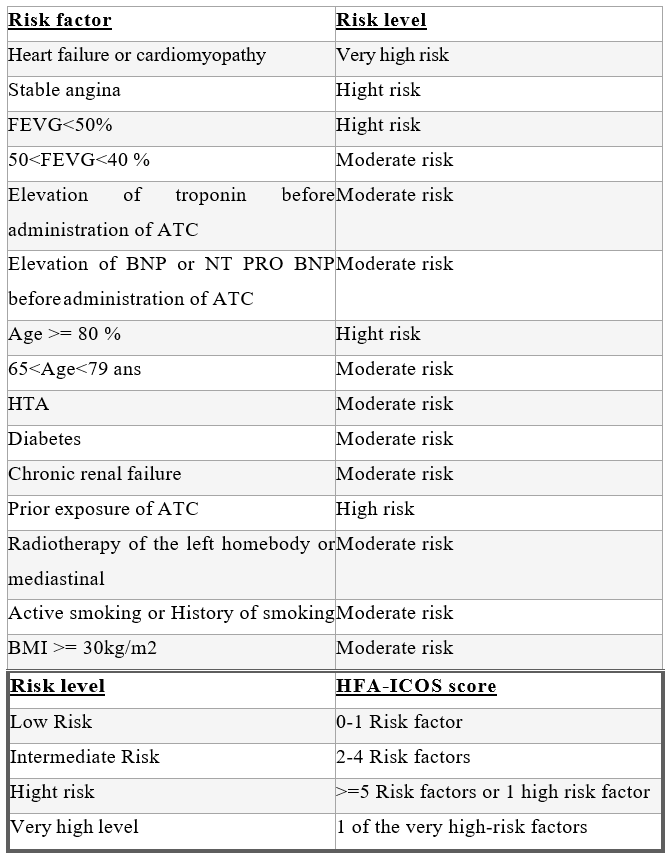
For patients scheduled to receive anthracyclines, evaluation before chemotherapy should include: 1-a clinical examination
- ECG (Class I),
- biomarker assay (Class I for patients at high risk of cardiotoxicity, IIa for low and intermediate risk) and a TTE (Class I).
- These recommendations reiterate the central role of TTE for the evaluation of left ventricular function, with measurement of LVEF, ideally in a 3D modality, and strain (Class I).
- Cardiac MRI should be proposed to the least echogenic patients (Class IIa), and isotopic EF should remain an examination of last resort if TTE or cardiac MRI does not allow a reliable measurement of LVEF (Class IIb).
At the end of this evaluation, 3 patient populations can be distinguished: a low-risk population, an intermediate-risk population and a high- or very high-risk population.
Although not included in this risk calculation, patients receiving a dose of doxorubin > 250mg/m² will be considered at higher risk of cardiotoxicity.
Prevention of anthracycline cardiotoxicity
In primary prevention, the ESC 2022 guidelines emphasize the importance of controlling cardiovascular risk factors (Class I) before, during, and after cancer treatment.
It is also recommended that patients at high and very high risk of cardiotoxicity (Class IIa) be treated with ACE inhibitors, beta-blockers and statins.
It is also possible to use dexrazoxane, an iron chelator that modulates the toxicity of anthracyclines on the myocyte in this same population (Class IIa).
In secondary prevention, patients with heart disease should be treated optimally, according to ESC recommendations (Class I).
In high- and very high-risk patients, a discussion between cardiologist and oncologist will focus on the possibility of using liposomal anthracyclines, which are less cardiotoxic than conventional anthracyclines (Class I).
Monitoring a patient on anthracyclines within one year of the introduction of TCAs
The monitoring regimen proposed by these ESC guidelines varies according to the patient's risk of cardiotoxicity.
In low-risk patients, a TTE should be performed 1 year after the end of treatment (Class I). Cardiac biomarker monitoring is not required (Class IIa).
In intermediate-risk patients, TTE should be performed after 250 mg/m² (Class IIa) and then 1 year after the end of treatment (Class I). Cardiac biomarkers should be measured every 2 courses and at 3 months after the end of treatment.
In high and very high-risk patients, monitoring will be reinforced with a TTE every 2 courses, and at 3 and 12 months after the end of treatment. Biomarker monitoring will be performed at every treatment cycle and at 3 and 12 months after the end of treatment (Class I).
Monitoring of a patient on anthracyclines beyond 1 year after the introduction of TCAs
Reassessment of patients who received anthracyclines will be done 1 year after the end of chemotherapy to decide on the monitoring regimen for patients who survived the cancer.
A patient who has received more than 250 mg/m² of doxorubicin, or a combination of doxorubicin and radiation therapy, and who has developed myocardial cardiotoxicity or heart disease during cancer therapy will be reclassified as high risk.
Annual screening for cardiovascular risk factors is recommended for all patients who have received cardiotoxic therapy.
In high-risk and very high-risk patients, this should be supplemented by cardiac ultrasound at 1, 3, and 5 years after the end of treatment, and then every 5 years (class IIa).
In intermediate-risk patients, a TTE could be discussed every 5 years (Class IIb).
These recommendations also emphasize monitoring of patients who received anthracyclines in childhood or adolescence, a population at high risk for cardiotoxicity.
In patients who have received 100-250 mg/m² of doxorubicin, or a combination of doxorubicin and radiation therapy, a TST should be performed every 5 years, in addition to annual screening for cardiovascular risk factors (Class IIa). It will be performed every 2 years in patients who received more than 250 mg/m² or a combination of radiotherapy and doxorubicin, with higher doses.
Treatment of anthracycline cardiotoxicity
In the event of anthracycline cardiotoxicity, management should include discussion of the possible resumption or permanent discontinuation of TCA therapy and the initiation of appropriate cardiological treatment (Figure 1).

Initiation of cardiological management
With regard to cardiological treatment, the ESC 2021 heart failure guidelines apply to patients with severe or moderate (class I) symptomatic or asymptomatic cardiotoxicity.
In case of moderate asymptomatic cardiotoxicity, an elevation of troponin, or a change in LMS, should lead to the introduction of treatment with ACE inhibitors and/or beta-blockers (Class IIa), with a low level of evidence.
On the other hand, the level of recommendation is lower in the case of isolated elevation of natriuretic peptides (Class IIb).
Discontinuation of cardiological treatment may be discussed in low-risk patients who have fully recovered normal cardiac function. For the other patients, treatment should be continued on a long-term basis.
Discussion of oncologic management modalities according to the severity of cardiologic toxicity
At the same time, anthracyclines will be discontinued in patients with severe or moderate cardiotoxicity, symptomatic or not, and will be discussed in cases of moderate symptomatic cardiotoxicity.
However, anthracyclines will be continued in patients with moderate asymptomatic cardiotoxicity (Class I) [8].
Conclusion
Cardio-oncology programs facilitate cancer treatment by minimizing unnecessary interruptions in cancer therapy.
The risk of CV toxicity is a dynamic variable. A baseline CV risk assessment is recommended for all cancer patients who are to receive potentially cardiotoxic cancer therapy.
Primary prevention of CV toxicity by cancer therapy is aimed at avoiding or minimizing the development of cancer treatment-related cardiovascular toxicity in patients without CVD.
Secondary prevention refers to interventions in patients with pre-existing CVD
Optimal management of cancer treatment-related cardiovascular toxicity and preexisting CVD is mandatory to facilitate cancer treatment and improve patient prognosis.
3D echocardiography, and cardiac biomarkers are provided to detect CV toxicity based on specific cancer therapies and baseline CV toxicity risk.
Patients should receive psychological support when needed and clear and accurate information about their condition to take an active role in managing their treatment and increasing compliance with cancer and CV therapies.
References
- Diehl V, Thomas RT, Re Hodgkin’s lymphoma-diag- nosis and treatment. Lancet, 2004; 5: 19-26.
- Glossmann JP, Jostig A, Diehl New treatments for Hodgkin’s disease. Curr Treat Option Oncol, 2002; 3: 283-290
- Campia U, Moslehi JJ, Amiri-Kordestani L, et Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Associa- tion. Circulation, 2019; 139: e579–e602.
- Hershman DL, Accordino MK, Shen S, et al. Association between nonadherence to cardiovascular risk factor medications after breast cancer diagnosis and incidence of cardiac Cancer, 2020; 126: 1541–1549.
- Senkus E, Jassem J. Cardiovascular effects of systemic cancer Cancer Treat Rev, 2011; 37(4): p. 300-311.
- Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and J Am Coll Cardiol, 2009; 53(24): p. 2231-2247.
- Floyd JD, et Cardiotoxicity of cancer therapy. J Clin Oncol, 2005; 23(30): p. 7685-7696.
- ESC guidelines cardio oncology.

