Sarcoidosis of the Breast
Zachery Lerch1, Oluwatunmike Ogedengbe1, Nicole D Serrant1, Courtney Short1, Shazia Zafar2, Sultan S Ahmed1,* and Syed A A Rizvi1,*
1Department of Biomedical Sciences, Ross University School of Medicine, Barbados
2Department of Biomedical Sciences, Southwest Florida Cancer Care, USA
3Department of Biomedical Sciences, College of Biomedical Sciences, Larkin University, USA
Received Date: 13/03/2023; Published Date: 30/05/2023
*Corresponding author:
- Syed A A Rizvi, MD, PhD, MPH, MBA, College of Biomedical Sciences, Larkin University, 18301 N Miami Ave, Miami, FL 33169, USA
- Sultan S. Ahmed, MD, College of Biomedical Sciences, Larkin University, 18301 N Miami Ave, Miami, FL 33169, USA
Abstract
Sarcoidosis is a chronic multisystemic inflammatory disease with an unknown etiology that is characterized by noncaseating granulomas in multiple organ symptoms. It is a rare disease with a US prevalence of 1 to 40 per 100,000 and an estimated worldwide prevalence of 50 to 160 per 100,000 people. Among these cases, it is commonly found in young to middle-aged women, especially those of African American or Northern European descent. Sarcoidosis primarily manifests in the lungs, representing 90% of the total number of cases. Isolated extrapulmonary manifestations represent 10% of all total cases and occur in the lymph nodes, spleen, liver, and skin in order of decreasing occurrence. Of that 10%, isolated sarcoidosis of the breast represents less than 1% of all cases. The diagnosis of sarcoidosis traditionally relies on three criteria to make a clinical diagnosis, such as suspicion based on imaging such as elevated Angiotensin-Converting Enzyme (ACE) levels and bilateral hilar adenopathy on chest radiography, noncaseating granulomas on biopsy of the nodule, and the exclusion of all possible diseases like fungal diseases or tuberculosis. In the case of breast sarcoidosis, which typically presents as a breast nodule, the diagnosis would follow the algorithm for breast lesions starting with a mammogram or ultrasound. However, irregular, or ill-defined spiculated masses on mammography, as well as a hypoechoic nodule on ultrasound, cannot rule out malignancy or benign conditions such as cysts or fibroadenomas. MRI has been found to be ineffective in providing a more definitive diagnosis. A biopsy showing noncaseating granuloma is necessary to lead towards sarcoidosis, but diseases showing similar histology in the breast such as tuberculosis are necessary to rule out before diagnosing breast sarcoidosis, just as in the criteria for pulmonary sarcoidosis. The treatment for sarcoidosis is case dependent, however the use of corticosteroid is common for significantly symptomatic patients presenting with pulmonary or serious extrapulmonary disease. Some complex cases may require immunosuppressive medication. In some cases where pharmacotherapy fails or does not stop the disease progression, it can lead to severe complications leading to heart or lung transplantation. Our patient is a 32-year-old female who has been suffering from multiple bilateral breast lumps with tenderness, erythema, and occasional exudative discharge from lesions for some time. She was without respiratory complaints such as shortness of breath or cough, denies family history of breast cancer and trauma. The most recent diagnostic mammography and ultrasound revealed 1.2 cm irregular hypoechoic and 2.0cm mass in the left breast. Ultrasound guided biopsy then revealed fibro-adipose tissue with stromal fibrosis associated with chronic inflammatory infiltrate without any no glandular elements. Chest x-ray and CT of lungs were not performed due to lack of pulmonary complaint and findings. She was found to be positive for the ACE gene deletion variant detected by PCR. A diagnosis of breast sarcoidosis is made, and the patient has been referred to an oncologist for further evaluation and management.
Introduction
Sarcoidosis is a disorder potentially involving multiple systems and characterized by a non-caseating granulomatous inflammation. Due to the variety of organs that may be involved, there are a multitude of symptoms that can occur. However, patients will have pulmonary involvement 95% of the time opposed having cutaneous involvement 15.9% of the time and lymph node involvement 15.2% of the time [1]. Patients will therefore most commonly present with respiratory symptoms such as dyspnea, cough and chest pain as well as weight loss, a fever and general malaise. When there is lung involvement, it typically will present as a diffuse interstitial lung disease, unfortunately with non-specific symptoms that are generally not helpful for diagnosis [2].
It should be noted that approximately one half of patients are diagnosed incidentally via chest radiograph indicating reticular opacities and bilateral hilar adenopathy that result in further evaluation of the patient. Regarding choices of imaging, a chest radiograph remains the best first test for a patient with suspected sarcoidosis, however it is not diagnostic. When radiographic findings are present, it is reasonable to send the patient for laboratory testing to further support a diagnosis [3]. Laboratory testing will reveal inflammatory markers for those who are experiencing acute sarcoidosis and elevated calcium, ACE, Alkaline phosphatase and decreased CD4+ T-Cells for those with chronic sarcoidosis [4]. ACE can be elevated in most patients with untreated sarcoidosis; however, it has poor sensitivity and specificity therefore is not usable for diagnosis, determination of disease progression or to prove efficacy of treatment [5].
While laboratory testing serves to narrow your differentials, biopsy of the lung tissue and lymph nodes remains the gold standard for diagnosis to this day. Biopsy is typically taken from the most easily accessible lesion and will show noncaseating granulomas under histopathologic analysis. Upon diagnosis, patients should be evaluated for the extent of disease involvement in additional organs. Notably, all newly diagnosed patients should have an ECG performed to assess for cardiac sarcoidosis. In addition to being the best initial imaging, chest radiographs are also useful for determining lung involvement which are broken into different Stages I-IV. Bilateral hilar adenopathy alone is characteristic of Stage I, addition of parenchymal involvement indicates Stage II. Parenchymal involvement without lymphadenopathy indicates Stage III. Finally, Stage IV is characterized by fibrosis which can progress in severity eventually leading to pulmonary hypertension in advanced disease, at this point patients may often require supplemental oxygen. It should be noted that radiographic staging does not serve as a marker of disease severity, instead serving as an indicator of anatomic involvement [6,7].
When considering management options for newly diagnosed patients, it is important to know the indicators for observation versus initiating the mainstay of treatment: glucocorticoids [7]. While radiographic staging doesn’t indicate disease severity, it can be utilized when determining when to start therapy. Asymptomatic patients with Stage I and II can be observed without therapeutic intervention as 60-80% of Stage I patients will have spontaneous remission and approximately 50% of Stage II patients will have radiographic resolution without treatment. Stage III patients should be closely observed without therapy, however 66% of these patients will eventually require some sort of medical intervention. As mentioned above, glucocorticoid therapy is the first line treatment of sarcoidosis. The exact mechanism is unknown; however, it is thought to play a role in decreasing the drivers of immune responses responsible for granuloma formation and eventual fibrosis. Indications to start glucocorticoids are concerning respiratory symptoms, decreased pulmonary function, pulmonary hypertension, and Stage IV radiographic changes (cavity formation, fibrosis with honeycombing) [7,8]. Dosing can vary; however, it is generally recommended to start therapy at 0.3-0.6 mg/kg of the patient’s ideal body weight, maintaining this dose for 4-6 weeks then re-assessing. Improvement of radiographic changes and pulmonary function can serve as an indicator to begin the tapering schedule which typically occurs in decrements of 5-10 mg every 4-12 weeks down to 10-15 mg daily. Maintenance therapy at this point should be carefully considered due to well-known adverse effects of long-term glucocorticoid therapy and the lack of formal evidence indicating its benefit [9,10].
Case Presentation
Patient is a 32-year-old female patient with a past medical history significant only for hypothyroidism who was seen in clinic with a complaint of a 2-year history of left breast inflammation and pain in the upper outer breast with a mass. Notably, she has no family history of malignancy and had previously been evaluated for left breast pain at the onset of the pain and subsequently underwent imaging and biopsy which revealed a neutrophilic cystic granulomatous mastitis. She was then referred to an infectious disease specialist but stated that she was unable to make an appointment due to the ongoing COVID-19 pandemic. She was seen two years later by breast and infectious disease specialists due to continued left breast pain and open skin lesions. She was instructed to follow closely, and that treatment wasn’t necessary unless there was malodorous discharge.
Her vital signs were within normal limits with weight of 197 pounds, BMI of 37.6, blood pressure of 125/75, pulse of 80, temperature of 96.8F, respiratory rate of 16 bpm, height of 61 inches. On examination, there was a movable mass palpated in the left breast, diagnostic mammography and ultrasound were ordered. Additionally, she was without systemic signs of infection or malignancy, being afebrile with no weight loss, general malaise or clubbing of the fingers. Her lung examination revealed the following: normal bilateral breath sounds upon auscultation, no respiratory distress, normal percussion, normal tactile fremitus.
On examination, there are multiple moveable superficial masses noticed in the left breast. Some of them are brownish-black and others are erythematous. There was exudative fluid noticed from the mass, with a serosanguinous fluid upon visual examination. There was no lymphadenopathy, nipple retraction, skin dimpling. The results of the most recent imaging showed a 1.2 cm irregular hypoechoic as well as a 2.0 cm mass in the left breast. Ultrasound guided biopsy then revealed fibro-adipose tissue with stromal fibrosis associated with chronic inflammatory infiltrate, of note, there were no glandular elements represented in the biopsy. Chest x-ray and CT of lungs were not performed due to lack of pulmonary findings that indicated lung involvement.
A CMP was then ordered which revealed cholesterol 226 mg/dL, HDL 56 mg/dL, Triglyceride 112 mg/dL, LDL 147 mg/dL, Sodium 138 mmol/L, Potassium 3.9 mmol/L, Chloride 102 mmol/L, Calcium 9.2 mmol/L Glucose 88 mg/dL, BUN 11 mg/dL, Creatinine 0.76 mg/dL and TSH 3.25 mIU/L. Hemoglobin 10.8 g/dL, Hematocrit 35.6%, MCV 77.4 fL, Platelet 262 thousand/uL, negative QuantiFERON TB Gold test, and positive for one copy of the ACE gene deletion variant via PCR.
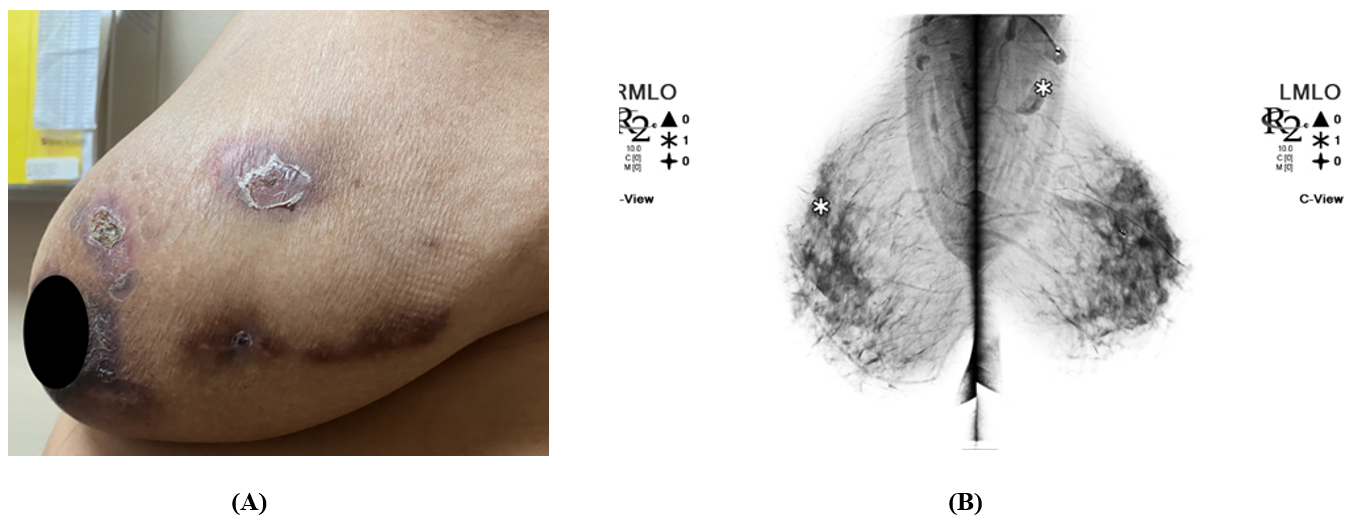
Figure 1. (A) Left breast exhibits mass, skin changes, and tenderness. Skin is warm, dry, and erythema of the left breast with crusted healing lesions. No inverted nipple or discharge. NB, patient gave verbal consent for the use of the images (B) Bilateral 3D Tomosynthesis Diagnostic Mammogram: Marker projects over the posterior left breast at the 2 o’clock position in the reported area of palpable lump. Underlying the marker, postsurgical changes with scarring and biopsy marker are seen 7cm from the left nipple. No architectural distortion, developing masses or suspicious clusters of microcalcifications are seen in the right breast.
Discussion
The patient’s bilateral erythematous, tender breast lumps, with exudative discharge from her breast lesions on further investigation, match the diagnosis of sarcoidosis of the breast. The 32-year-old female patient is in the relevant age and gender group for sarcoidosis but given that the patient’s symptoms and the imaging showing hypoechoic masses on ultrasound suggested a benign disease such as mastitis with the risk of malignancy, further investigation was necessary [11]. The ACE gene polymorphism, specifically a gene deletion, leads us to believe that sarcoidosis of the breast is the most likely diagnosis. ACE gene polymorphism indicates a deletion is associated with elevated ACE levels, which are commonly found with sarcoidosis, but neither elevated ACE levels nor the polymorphism are specific enough for a diagnosis of sarcoidosis [12]. Given the patient's lack of pulmonary symptoms such as coughing or shortness of breath, it is unlikely to be an extension of pulmonary sarcoidosis.
While sarcoidosis is a disease found across the world and in all populations, studies have found that certain groups of people along racial, ethnic, and gender lines are more affected than others. Focusing on the most recent studies across the world from 2015 to 2017, Northern Europeans and African Americans tend to have a higher incidence and prevalence of sarcoidosis compared to other ethnic and racial groups, and the age of disease onset is close to 30 to 50 years old [12-17]. While incidence and prevalence are variable among the sexes, women are more likely to have the disease than men, and while there are certain nuances and limitations within the reported data, the general trends in current data are significant enough to warrant further study.
Observations of the African American and North European prevalence of sarcoidosis have been made through several recent studies. Using the Optum Health care database for US (United States) data, African Americans had a larger incidence and prevalence compared to Caucasians, the second largest, with Hispanics and Asians having the lowest prevalence and incidence [14]. Further into the data, not only are African Americans found to have a higher incidence, but African American women also have the highest sarcoidosis prevalence compared to men or women of any race [14]. Moving outside of the US for rates of people with African ancestry, the French study focusing on hospitals in a multicultural Seine-Saint-Denise country showed Afro-Caribbeans had a higher incidence of sarcoidosis of 16.9 per 100,000 per year, similar but less than 17.8 per 100,000 per year in the US study, than North Africans of 9.7 per 100,000 per year [14, 16]
When comparing diverse groups of Europeans in terms of sarcoidosis prevalence and incidence, Sweden’s study using a nationwide population database showed 11.5 per 100,000 per year and a prevalence of 0.16%, showing a greater prevalence than Italy's study which had a prevalence of.005%, and Europeans in France which showed an incidence of 2.4 per 100,000 per year [12, 16]. However, in the French study, it is not clear whether the Europeans are all French or not [16]. Despite the limitations of the numerous studies for sarcoidosis epidemiology, such as using a health company's limited database for the US population instead of a more generalized sampling of the population, and missing data on sarcoidosis in African, Hispanic, Asian, and Middle Eastern countries, it is a good overview of current general trends.
Based on sex and age in sarcoidosis epidemiology, the studies tend to be inconsistent with recent tend to show women having a higher prevalence and incidence than men in France, Italy, and Taiwan, but the study in Sweden showed men having a greater incidence of sarcoidosis [12, 15, 16, 17). In terms of age at diagnosis, the average age of diagnosis in most studies was between 47 and 51 years old [13]. However, there tends to be an age gap between the age of onset of sarcoidosis among the sexes, with women being younger than men. The greatest gap in the current data on the age of onset of sarcoidosis was seen in Sweden's study which showed the onset on average was 45 years old in men and 54 years old in women, a more than 10-year difference [12]. More studies with a wide population sampling may be needed to see the full trend among the different sexes and ages across the world, but age at disease onset appears to trend around a distinct time within the 5th and 6th decade.
Despite incidence of sarcoidosis on sex is not as consistent as the racial or age trends around the world, current epidemiology studies on sarcoidosis show specific trends such as Northern Europeans and African Americans having the greatest incidence and prevalence, age of onset on average being between 40-50 years old with women tending to be younger than men. Even though the general trends are important for evaluating elevated risk groups, the shortcomings of how the studies were done, a specific hospital or insured population rather than the general population being studied, and certain races, ethnic groups being excluded should be investigated in the future to see how the trend changes with more information.
The purpose of this study is to present the case of a woman where the first clinical manifestation of sarcoidosis occurred in the breast. Given the rarity of finding a primary sarcoidosis manifesting in breast first, it may be misdiagnosed as a malignancy. The patient is currently at the diagnostic stage, with specialists on board throughout the treatment….
Disclosures: The authors report no relevant financial relationships.
References
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med, 2001; 164(10 Pt 1): 1885-1889. doi:10.1164/ajrccm.164.10.2104046.
- Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med, 2015; 109(2): 272-278. doi:10.1016/j.rmed.2014.12.012.
- Sawahata M, Yamaguchi T. Imaging Findings of Fibrosis in Pulmonary Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis, 2022; 39(2): e2022018. doi:10.36141/svdld.v39i2.12995.
- Selroos OB. Biochemical markers in sarcoidosis. Crit Rev Clin Lab Sci, 1986; 24(3): 185-216. doi:10.3109/10408368609110273.
- Baudin B. Angiotensin I-Converting Enzyme (ACE) for sarcoidosis diagnosis. Pathol Biol, 2005; 53(3): 183-188. doi:10.1016/j.patbio.2004.09.003.
- Wessendorf TE, Bonella F, Costabel U. Diagnosis of Sarcoidosis. Clin Rev Allergy Immunol, 2015; 49(1): 54-62. doi: 10.1007/s12016-015-8475-x.
- Ungprasert P, Ryu JH, Matteson EL. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin Proc Innov Qual Outcomes, 2019; 3(3): 358-375. doi: 10.1016/j.mayocpiqo.2019.04.006.
- Brito-Zerón P, Pérez-Alvarez R, Pallarés L, et al. Sarcoidosis: an update on current pharmacotherapy options and future directions. Expert Opin Pharmacother, 2016; 17(18): 2431-2448. doi:10.1080/14656566.2016.1258061
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J, 2021; 58(6): 2004079. doi:10.1183/13993003.04079-2020.
- Judson MA. The treatment of sarcoidosis: translating the European respiratory guidelines into clinical practice. Curr Opin Pulm Med, 2022; 28(5): 451-460. doi:10.1097/MCP.0000000000000896.
- Kim YR, Kim HS, Kim HW. Are Irregular Hypoechoic Breast Masses on Ultrasound Always Malignancies? A Pictorial Essay. Korean J Radiol, 2015; 16(6): 1266-1275. doi: 10.3348/kjr.2015.16.6.1266.
- Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J, 2016; 48(6): 1690-1699. doi: 10.1183/13993003.00477-2016.
- Arkema EV, Cozier YC. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis, 2018; 9(11): 227-240. doi: 10.1177/2040622318790197.
- Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc, 2016; 13(8): 1244-1252. doi: 10.1513/AnnalsATS.201511-760OC.
- Beghè D, Dall'Asta L, Garavelli C, Pastorelli AA, Muscarella M, Saccani G, et al. Sarcoidosis in an Italian province. Prevalence and environmental risk factors. PLoS One, 2017; 12(5): e0176859. doi: 10.1371/journal.pone.0176859.
- Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, Sanyal S, Brillet PY, Brauner M, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J, 2017; 50(2): 1602419. doi: 10.1183/13993003.02419-2016.
- Wu CH, Chung PI, Wu CY, Chen YT, Chiu YW, Chang YT, et al. Comorbid autoimmune diseases in patients with sarcoidosis: A nationwide case-control study in Taiwan. J Dermatol, 2017; 44(4): 423-430. doi: 10.1111/1346-8138.13654.

