T-Cell Large Granular Lymphocytic Leukaemia in a South African Patient Living with HIV
Q van Staden1,*, J Bailly2 and V J Louw1
1Division of Clinical Haematology, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
2Division of Haematology, Department of Pathology, Faculty of Health Sciences, University of Cape Town and National Health Laboratory Service, Groote Schuur Hospital, South Africa
Received Date: 25/02/2023; Published Date: 02/05/2023
*Corresponding author: Quintin Andre van Staden, Division of Clinical Haematology, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
Abstract
A middle-aged South African living with Human Immunodeficiency Virus (HIV) presented with constitutional symptoms and was empirically treated for Tuberculosis (TB). A bone marrow biopsy performed to investigate a severe bicytopenia revealed findings in keeping with T cell large granular lymphocytic leukaemia. Co-existing TB infection was never proven. Over the course of treatment, the patient developed Kaposi sarcoma with gastro-intestinal bleeding, significant calcineurin inhibitor-related side effects and severe sepsis. Due to recurrent drug-related events, treatment for T-LGLL was stopped and the decision was made to provide supportive care only. A year later, the patient is clinically well and has a laborious occupation despite an increasing absolute lymphocyte count. This case report highlights some of the challenges faced when treating people living with HIV and diagnosed with a haematological malignancy.
Key words: T-cell large granular lymphocytic leukaemia (T-LGLL), HIV
Background
T-cell large granular lymphocytic leukaemia (T-LGLL) in the context of human immunodeficiency virus (HIV) infection, was first described in 1997 [1]. Since then, a handful of case reports have been published, none of which (as far as could be ascertained) details a case that reflects an African or South African demographic and management approach.
Case Presentation
A 46-year-old South African male was referred to the Clinical Haematology Unit at a tertiary hospital in Cape Town, South Africa. He was diagnosed with HIV in 2007 and was adherent to Antiretroviral Treatment (ART). He had an absolute CD4 count of 273 cells/uL and a HIV Viral Load (VL) of 22 copies/mL. A few weeks prior, he presented to the Emergency Unit of a secondary hospital with a 5-week history of petechial rash and 3-week history of a cough, unintentional weight loss, night sweats, fatigue, intermittent haematemesis, haemoptysis and melaena stools. He was noted to have marked pallor and a widespread petechial rash.
Investigations
The initial laboratory work-up for this patient (Table 1) revealed a severe iron deficiency anaemia (haemoglobin [Hb] 1.7 g/dL), severe thrombocytopenia (platelet count 5 x 109/L), a normal coagulation screen and a lymphocytosis (absolute lymphocyte count [ALC] 12.53 x 109/L).
A haemolytic screen showed a positive polyspecific direct antiglobulin test but no laboratory evidence of haemolysis. A serum Hepatitis B surface antigen, rheumatoid factor and anti-cyclic citrullinated peptide test were negative. A serum vitamin B12 level was normal. A gastroscopy revealed Helicobacter pylori associated gastritis with no active bleeding.
A chest X-ray showed hilar adenopathy (Figure 1), suggesting either Mycobacterium Tuberculosis (TB) infection or a malignancy. A Computerized Tomography (CT) scan of his chest demonstrated bilateral lung base ground glass opacification along with nodularity and bronchiectatic changes within the left lower lobe (Figure 2), strengthening a diagnosis of TB.
Multiparameter flow cytometry performed on peripheral blood, revealed an expanded T-cell population with a CD3+CD8+CD56+CD57- immunophenotype and aberrant loss of CD5 and CD7 (Table 2 and Figure 3A-3C). A polymerase chain reaction-based T-cell receptor gamma chain gene rearrangement study revealed a clonal T-cell population. T-LGLL was favoured based on the immunophenotypic findings. The initial bone marrow biopsy (BMB) supported a diagnosis of T-LGLL and showed a hypercellular marrow with a 35% tumour burden (Table 1). There was an interstitial and intra-sinusoidal increase in CD3+CD8+ lymphocytes and markedly disordered megakaryopoiesis.
A repeat pre-treatment BMB was requested to perform molecular studies and to exclude an associated myelodysplastic syndrome. This revealed an increase in small-to-intermediate sized lymphoid cells with variable amounts of weakly basophilic cytoplasm and fine azurophilic cytoplasmic granules (Figure 4A). The megakaryocytes were decreased with hyperchromatic degenerate forms noted, in keeping with HIV-associated cytopathy (2). The repeat BMB (Figure 4B-4C) confirmed the diagnosis of T-LGLL.

Figure 1: Chest X-ray showing hilar adenopathy (red arrow).

Figure 2: CT scan demonstrating ground glass opacification, nodularity and brochiectactic changes in the left lower lobe (black arrow).
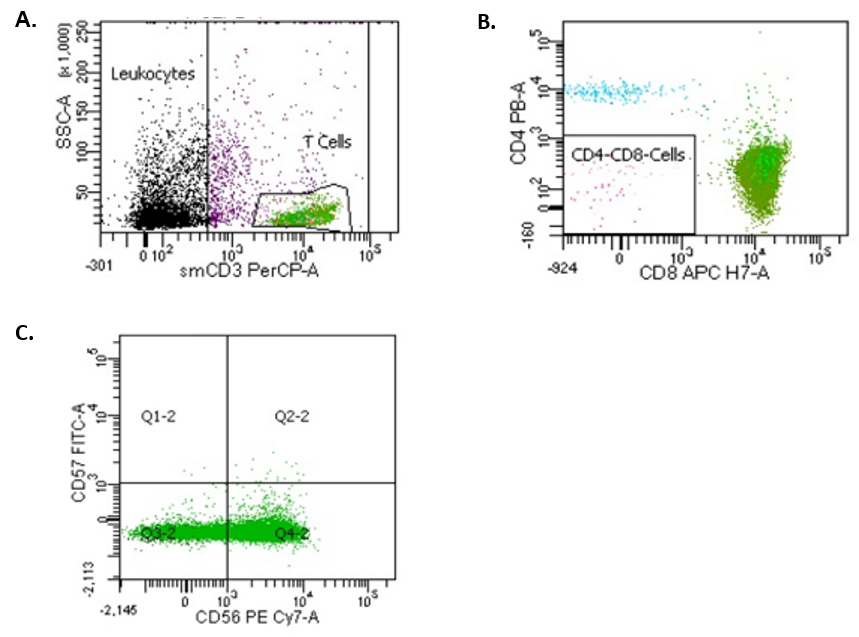
Figure 3: Flow cytometric analysis. Panel A shows side scatter (SSC) versus surface CD3. T cells are gated for further analysis (green). Panel B shows an expanded CD8+ population (green). Panel C shows a range of CD56 expression and absence of CD57 expression in the CD3+CD8+ population.
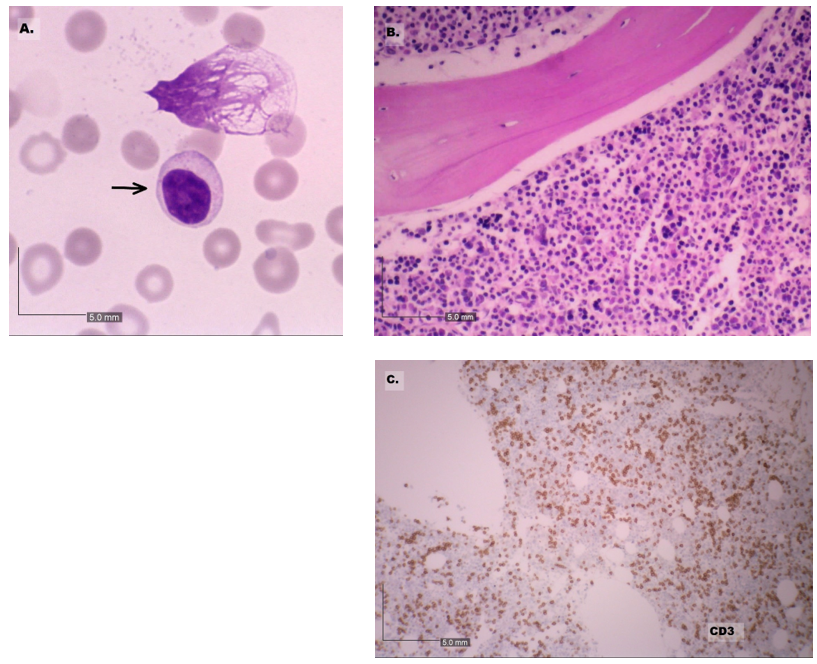
Figure 4: The peripheral blood with circulating abnormal lymphoid cells (arrow) and frequent smear cells. 100x magnification (panel A). The bone marrow biopsy revealed an increased in CD3+ lymphoid cells. 10x magnification (panels B and C).
Table 1: Patient blood results.
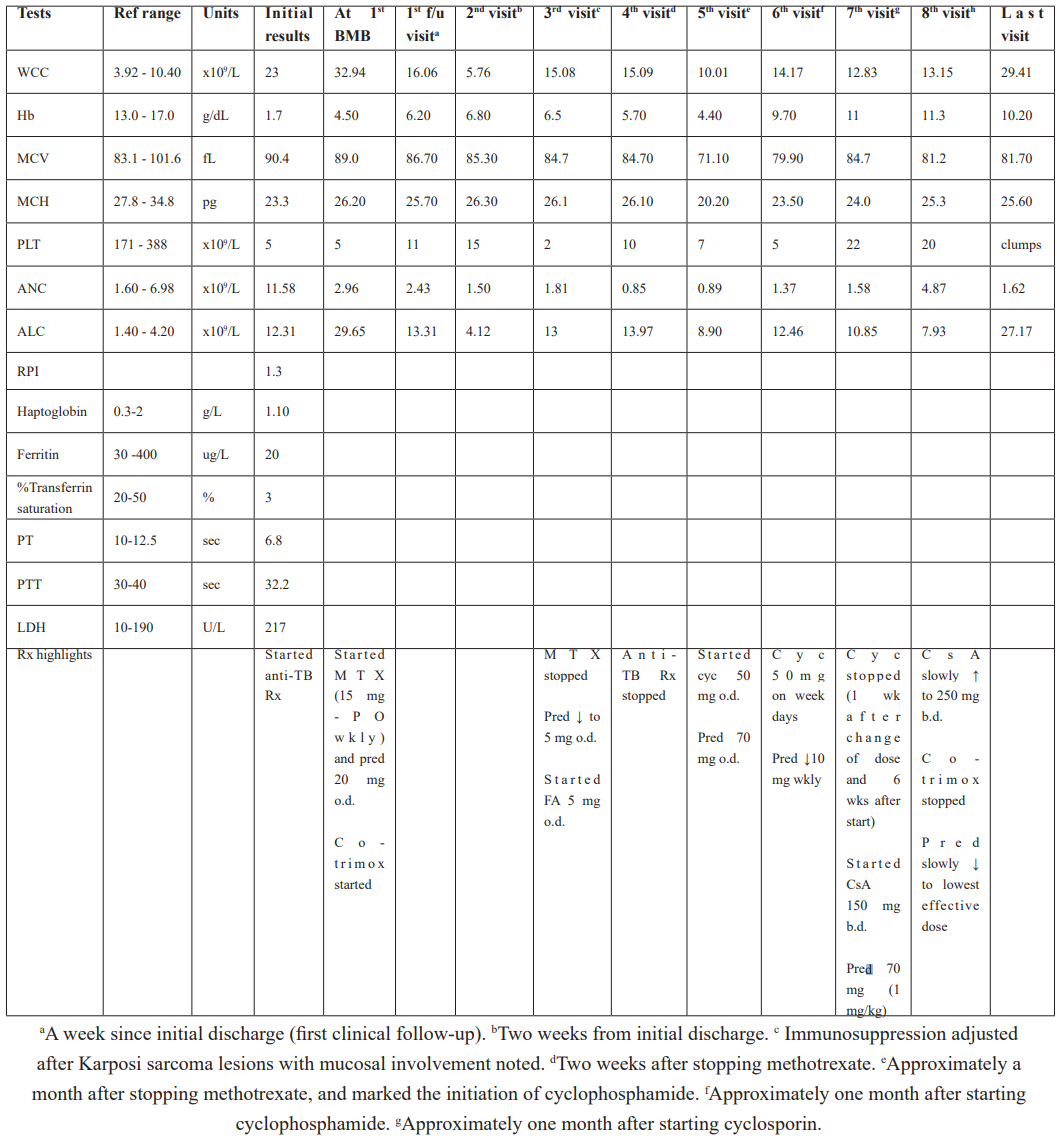
Ref, reference; F/u, follow-up; WCC, white cell count; Hb, haemoglobin; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; PLT, platelet; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; RPI, reticulocyte production index; PT, prothrombin time, PTT, partial thromboplastin time; sec, seconds; LDH, lactate dehydrogenase; BMB, bone marrow; Rx, treatment; Erta, ertapenem; Wk, week; Co-trimox, co-trimoxazole; MTX, methotrexate; Pred, prednisone; PO, per os; FA, folic acid; o.d., once daily; b.d., twice a day; Cyc, cyclophosphamide; CsA, ciclosporin.
Table 2: Flow cytometry findings at presentation.

Treatment, Follow-Up and Outcome
The patient was empirically started on anti-TB treatment. A repeat CT showed no features of active TB and TB treatment was stopped after three months.
The patient was started on Methotrexate (MTX) 15 mg per os weekly, folic acid 5 mg daily, prednisone 20 mg daily and co-trimoxazole prophylaxis. Within two weeks of treatment, he developed skin and mucosal Karposi Sarcoma (KS). MTX was interrupted and the prednisone dose was decreased to 5 mg daily. Two weeks after stopping MTX, he presented with peripheral neuropathy, an increasing ALC, worsening anaemia and thrombocytopenia and decrease in Mean Corpuscular Volume (MCV) (Table 1). Intravenous iron dextran complex (1000 mg) and a packed red cell infusion were given due to blood loss from gastrointestinal KS.
Within one month, the KS lesions completely resolved. In view of a persistently raised ALC and worsening anaemia and thrombocytopenia, cyclophosphamide 50 mg daily was started and the prednisone dose was increased to 1 mg/kg. He initially had a modest response. A month after the cyclophosphamide was introduced, it was reduced to 50 mg on weekdays and his prednisone was weaned by 10 mg weekly.
A week after the above dose adjustments, he presented, with petechiae and mucosal ecchymoses. He had a severe thrombocytopaenia (5 x 109/L), and an increase in his ALC (Table 1). After almost 6 weeks of cyclophosphamide use and no evidence of improvement, it was decided to change to cyclosporine (CsA) 150 mg twice a day (b.d.) and to increase the prednisone dose to 1 mg/kg.
He subsequently followed-up on a near weekly basis for CsA levels. He continued to require platelet transfusions. A month after starting CsA, the patient had a stable Hb (between 11.0 and 11.3 g/dL), improving platelet count (Table 1) and was clinically well. The CsA dose was slowly titrated to 250 mg b.d. The prednisone was slowly weaned to 20 mg daily.
A month later he was admitted with a gluteal abscess-related methicillin-sensitive Staphylococcus aureus sepsis. He was treated with Intravenous (IV) cefazolin and was switched to a renal friendly ART. The admission was further complicated by CsA-induced acute pancreatitis, a hyperosmolar hyperglycaemic state and septicaemic shock. Once discharged from the intensive care unit, he developed Clostridioides difficile-induced diarrhoea and Klebsiella pneumoniae sepsis for which he received oral vancomycin and IV tigecycline. Due to excessive toxicity, it was decided to discontinue treatment for the T-LGLL and only provide supportive care.
In the following 11 months after discharge, his absolute CD4 count remained >200 cells/uL and his HIV VL remained suppressed. His white cell count doubled in a period of 6 months due to a consistently increasing ALC (Table 1). Clinically, he had no lymphadenopathy nor splenomegaly, but reported occasional fever and night sweats.
Discussion
Case reports of PLHIV and T-LGLL describe patients presenting at a younger age [3,4] with males and females equally affected [4,5]. T- LGLL usually follows an indolent clinical course, although an aggressive variant has been described [6]. Patients more commonly present with a cytopenia-related complications and up to 35% of patients have splenomegaly [7,8].
Up to 90% of cases show a CD3+CD8+CD16+CD57+TCR-αβ+ immunophenotype [9]. Although the expression of CD56 is uncommon in T-LGLL, a CD56+CD57- variant has been described in case reports [10].
Purine analogues form part of second-line therapy [11] but can cause depletion of CD4+ T cells [12]. This further increases the risk of infections and complications associated with a low CD4+ count [13-15].
Acknowledgements
Dr Q. van Staden: Primary author, substantial contribution to drafting the paper, critical revision and approval to submit.
Dr J. Bailly: Substantial contribution to drafting the paper, critical revision, Haematopathology input and insight, image selection and approval to submit.
Professor V. Louw: Substantial contribution to drafting the paper, critical revision, clinical input and insight and approval to submit.
Conflicts of interest: None
None of the authors were funded to produce this case report.
Patient’s Perspective
The patient gave his written consent for the authors to write and publish this case report.
He expressed extreme gratitude and thanks to the medical team and reported that he is doing well. When reflecting on his experiences, he expressed how overwhelmed and at a loss he felt, particularly during his first admission. He specified the Haematology team as being pivotal in supporting him, guiding him through each step in the process. He underlined how he and his family valued the open channels of communication and appreciated the honest, understandable updates. He feels as if he can, for now, lead a full life enabling him to take on physically demanding work for his livelihood.
References
- Pulik M, Lionnet F, Genet P, Petitdidier C, Jary L, Fourcade C. CD3+ CD8+ CD56- clonal large granular lymphocyte leukaemia and HIV infection. Br J Haematol, 1997; 98(2): 444–445.
- Bain BJ. The haematological features of HIV infection [Internet]. Vol. 99, British Journal of Haematology. Blackwell Publishing Ltd, 1997; p. 1–8.
- Kronenberg A, Seebach JD, Bossart W, Weber R. Polyclonal proliferation of large granular lymphocytes during cytomegalovirus primary infection in a human immunodeficiency virus-infected patient receiving antiretroviral therapy. Clin Infect Dis, 2001; 33(5): 34–36.
- Rose A, Isenalumhe L, Bergh M Van den, Sokol L. Clonal T-cell large granular lymphocytic disorders manifesting in patients with HIV-1 Infection: Case Series and review of the literature. Mediterr J Hematol Infect Dis, 2018; 10(1): 6–10.
- Shah MV, Hook CC, Call TG, Go RS. A population-based study of large granular lymphocyte leukemia. Blood Cancer J, 2016; 6(8): e455.
- Alekshun TJ, Tao J, Sokol L. Aggressive T-cell large granular lymphocyte leukemia: A case report and review of the literature. Am J Hematol, 2007; 82(6): 481–485.
- Neben MA, Morice WG, Tefferi A. Clinical features in T-cell vs. natural killer-cell variants of large granular lymphocyte leukemia. Eur J Haematol, 2003; 71(4): 263–265.
- Lamy T, Loughran TP Jr. Clinical features of large granular lymphocyte leukemia. Semin Hematol, 2003; 40: 185.
- Sokol L, Loughran TP. L eukemias Large Granular Lymphocyte Leukemia, 2006; 263–73.
- Brugnoni D, et al. The primary response to HIV infection is characterized by an expansion of activated CD8+ CD28− cells. Aids, 1996; 10(1): p. 104-105.
- Fortune AF, Kelly K, Sargent J, et al. Large granular lympho- cyte leukemia: natural history and response to treatment. Leuk Lymphoma, 2010; 51: 839–845.
- Samonis G, Kontoyiannis DP. Infectious complications of purine analog therapy. Curr Opin Infect Dis, 2001; 14(4): 409–413.
- Gazitt T, Loughran TP. Congenital and Acquired Neutropenia. Chronic neutropenia in LGL leukemia and rheumatoid arthritis, 2017; 181–186.
- Lamy T, Moignet A, Loughran TP. LGL leukemia: From pathogenesis to treatment. Blood [Internet], 2017; 129(9): 1082–1094. DOI: http://dx.doi.org/10.1182/blood-2016-08-692590
- Moignet ALT. Latest Advances in the Diagnosis and Treatment of Large Granular Lymphocytic Leukemia. Am Soc Clin Oncol Educ B, 2018; 23(38): 616–625.

