The Importance of Environmental Factors in Prostate Biopsy Related Infection
Raghav Varma1,*, Yew Fung Chin2, Rayvind Purushothaman2, Yen Nee Jenny Bo2, Guleed Mohamed2, Jasreen Dhother2, Julia Cheetham2, Avril O’Gorman2 and Andrew Elves2
1Department of Urology, Hereford County Hospital, Herefordshire, UK
2Department of Urology, Royal Shrewsbury Hospital, Shropshire, UK
Received Date: 31/01/2023; Published Date: 10/03/2023
*Corresponding author: Raghav Varma, Department of Urology, Hereford County Hospital, Herefordshire, United Kingdom
Abstract
Introduction: Control of sepsis related complications with TRUS biopsy focuses upon antibiotic prophylaxis. We report the findings from an outbreak investigation related to an environmental pathogen. This identifies the importance of infection control practices that extend beyond antibiotic prophylaxis and may have applications for trans perineal practice.
Methods: Retrospective and prospective analysis of the electronic records from a single urology centre, servicing a group of urologists. Data collected over two consecutive audit loops between December 2020 and September 2021.
Results: The exogenous pathogen S. marcescens was identified as the causative organism. A number of infection control interventions were put in place; establishing robust PPE and hand washing protocols, correct disinfection of the room and ultrasound machine between cases, correct use of ventilation, limiting the number of people in the procedural room and changes to equipment. A total of 274 cases were performed, 137 cases following the implementation. Analysis revealed a significant improvement in the rate of sepsis related admissions improved from 9.5% to 0.7% following the introduction the Infection Prevention and Control (IPC) interventions. There was no change to the antibiotics used.
Conclusions: With IPC policy we have demonstrated a significant improvement in the rate of sepsis, reducing readmissions and patient morbidity. Environmental pathogens may explain the significant differences in rate of sepsis following TRUS biopsy within literature and be considered in service design for prostate biopsies.
Introduction
Transrectal ultrasound (TRUS)-guided procedure is the most common procedure in obtaining histology to diagnose prostate cancer worldwide [1]. Since its introduction of systemic sextant biopsy in 1989 [2], millions of TRUS biopsies have been performed around the world [3].
The procedure involves 12 or more needles passing through the rectal mucosa into adjacent prostate to obtain histology. Given this non-sterile procedure, despite prophylactic antibiotics prior to the procedure sepsis is a common complication due to contamination from rectal flora by direct inoculation from the biopsy needle [4]
Sepsis rates after TRUS biopsy ranges from 1% to 2%, as stated in British Association of Urological Surgeons (BAUS) Limited TRUS Biopsy Consent forms [5]. A systematic review including 165 studies with 162,577 patients described sepsis rates of 0.1% and 0.9% for transperineal and transrectal biopsies, respectively [6]. With 10% of patients with sepsis requiring high dependency care or intensive care unit admission [7]. However, despite antimicrobial prophylaxis there are reports of much higher incidence of sepsis in the literature, with the rates as high as 9.4% [8-10].
Within our institution where the readmission rate is regularly audited, we noted a rapid rise in the readmission rate from sepsis from a baseline of 2% to 10% over a three-month period. We report the findings of the multidisciplinary outbreak investigation and the application of the findings to prostate biopsy procedures generally.
Aims
To investigate the possible cause of an infection outbreak in a transrectal prostate biopsy service and implement appropriate remedial actions to reduce the sepsis readmission rate.
Patient & Methods
Between April and May 2021 our TRUS Biopsy service was stopped for the immediate need to understand the origin of the increased rate of sepsis and examination of the standard practice within the service to reduce risk and prevent harm. A multidisciplinary team was created comprising microbiology, infection control and clinical staff with involvement of the hospital executive team. The admissions were reviewed with regard to the operating surgeon, antibiotic prescribing, dosing and administration timing with respect to the biopsy. Urine and blood cultures were reviewed. A rigorous assessment of the procedure environment, infection control procedures and disinfection procedures were conducted. Faults were identified, remedial actions taken with regard to the procedure room organisation and procedures to facilitate good infection control practice (Table 1).
Clear criteria for restarting and the continued performance surveillance of the service were agreed, with a view of auditing to show the return to baseline rate of sepsis. Under the assumption of a readmission rate with sepsis to drop from the current rate of 10% to the upper range of BAUS Limited TRUS Biopsy Consent forms 2% (5), B error of 0.15 and alpha error of 0.05, 2 sided, 137 participants were required in each group.
Retrospective and prospective data collections for all patients underwent TRUS biopsy for the period between December 2020 and September 2021 were analysed.
The primary objective was to compare re-admission rates prior to the service review and after implementation of the changes. Patients who had undergone prostate biopsies needed to fulfil at least two of the following criteria: fever >38.0°C or hypothermia <36.0°C, tachycardia >90 beats/minute, tachypnea >20 breaths/minute, leucocytosis >12*109/l or leucopoenia <4*109/l (SIRS criteria) within 14 days of procedure were regarded as post TRUS biopsy sepsis [11].
The first part of our two-part audit was a retrospective study conducted over four months from December 2020 to April 2021 where 137 cases were identified. The second part of the audit was a prospective study conducted over five months from May 2021 to September 2021 with 137 cases identified. Patient demographics, indication, histology, antimicrobial therapy and outcomes measures such as: hospital readmission, microbial culture results, length of stay, intensive care unit admission and adverse events were all digitally collected.
To account for potential confounding factors for urinary infection post biopsy, we conducted student t-test using SPSS Version 20 (IBM SPSS Statistics, Armonk, NY, USA). The variables included: age, American Society of Anaesthesiologusts’ (ASA) classification, performance status, prostate size, previous urine analysis and antibiotic prophylaxis.
All patients involved were informed of the assessment of the service in response to the sepsis events. They were contacted following the analysis and informed of the findings and changes made.
Table 1

Results
A total of 274 patients were included in this audit cycle. The demographics of the first and second audit are shown in Table 2. There were no statistical differences in demographics between the two groups.
In the first audit 13/137 (9.5%) patients re-presented to Accident and Emergency (A&E) meeting the SIRS criteria with suspected post TRUS biopsy sepsis. Of the 13 patients re-admitted, 3 had positive blood cultures for E.Coli and 4 had positive blood cultures for Serratia Maecescens. They were all managed with single dose of 5mg/kg intravenous (IV) gentamicin and continued IV meropenum. The average length of stay was 5 days ranging from 3 to 10 days, with no ITU admissions.
In the second audit 1/137 (0.7%) patients re-presented to A&E meeting the SIRS criteria with suspected post TRUS biopsy sepsis. There was no growth in urine or blood culture, managed with IV meropenum and single dose of 5mg/kg IV gentamicin cover, with a 5-day length of stay, and no ITU admission.
The difference in sepsis rates between audits is statistically significant, a p value of 0.0025, utilising chi squared test, with Yates’s correction.
In both our audits we had one patient return secondary to retention requiring catheterisation, no other complications were noted for either of our study groups. Three patients in the first audit were re-listed due to a UTI, and underwent biopsy once cleared. Five patients in the second audit were re-listed due to a UTI, and underwent biopsy once cleared. One patient was excluded from both audits due to change of preference to transperineal biopsy.
Table 2

Discussion
Our study showed a significant improvement in the rate of sepsis post TRUS biopsy by implementing a structured and systematic approach to reduce the risk of bacterial contamination when conducting TRUS biopsy. We were able to reduce our rate of sepsis from 9.5% to 0.7% with strict adherence to infection control and formulation of infection prevention practices.
The prevalence of sepsis following TRUS biopsy varies significantly in the literature, with the lowest ranges from 0% [12] to the highest ranges of 9.4% [9]. Multiple explanations have been put forward; antibiotic prophylaxis used, prebiopsy bowel preparation, formalin disinfection, number of sampled cores and prior antibiotic exposure [13]. A recent systematic review found none but antibiotic prophylaxis used to be significant in affecting the rate of sepsis [13]. The increase in our initial cohort of patients was related to Serratia marcescens. It is an opportunistic, gram negative, nosocomial pathogen which belongs to Enterobacteriaceae family [14]. One causality that is lacking in the literature is the roll of simple infection prevention practise.
Route cause analysis was undertaken examining the procedure environment, Infection Control Policy (ICP) practice as well as the standard operating procedure. This identified a number of risk factors. Inconsistent use and understanding of the purpose of the air conditioning facility. Poor hand hygiene and PPE practice. Inconsistent understanding of good decontamination practice leading to variation in practice. Room layout not supporting good ICP practice through difficult access to sinks and bins. Lack of a cleanable storage facility for invasive equipment (Figure 1).
Simple measures such as hand washing multiple times during the procedure, donning and doffing of personal protective equipment, appropriate storage of invasive equipment, well ventilated and thorough cleaning of the room and equipment significantly reduced our rate of sepsis following TRUS biopsy.
These changes came when it was recognised that the limitation was not prophylactic antibiotic or our management of pre-biopsy urine infection. After multiple discussions with the microbiologists, all patients were commenced on triple antibiotic prophylaxis; 5mg/kg intravenous gentamicin, 500mg oral Ciprofloxacin and 400mg rectal Metronidazole. Any patient with positive pre-biopsy urine analysis was rescheduled, ensuring the infection was cleared prior to biopsy. However, despite these changes the rate of sepsis remained high at 10%, in our local audit. With the help of clinical quality commission, infection control deficiencies were identified and rectified to help improve the rate of post biopsy sepsis.
Current NICE and EAU guidelines do not distinguish between LATP and TRUS Biopsy for first line biopsy [15,16] Trans perineal biopsy sepsis rates are extremely low, regardless of the use or omission of prophylactic antibiotics. Recently there has been a move towards local TP biopsies, with a multicentre prospective outcome analysis of 1218 patients showing a sepsis rate of 0.16% [17]. Easy disinfection of the perineal skin and avoidance of transrectal trocar passage has decreased the rates of sepsis below 0.5% with some studies publishing zero rates of sepsis [18]. With transperineal biopsy there is limited opportunity for contamination from rectal flora by direct inoculation from the biopsy needle, the cause of sepsis may be secondary to environmental pathogens and avoided by following strict ICP policy. Foundation research conducted by Thompson et al in 1982 found skin contaminants as the most common cause for transperineal biopsy related bacteraemia [19]. With Werneberg et al. showing E.Coli to have little role in TP biopsies [20].
A recent review in the role of prophylactic antibiotics in transperineal biopsy found there were 19/37 805 (0.05%) episodes of sepsis in the group of men who received antibiotics, which was similar to the no antibiotic group with 4/4772 (0.08%) episodes (p = 0.2) [21]. This suggests that the antibiotics used may not cover the causative organisms and that these infections may be due to the variation in ICP. Standard operating procedures should define the environment set up, equipment storage, ICP stents with regards to hand hygiene, use of PPR and decontamination.
It is important to note that the first audit was retrospective study by design and only the second audit data was collected prospectively. Another limitation included different clinicians conducting the procedure. However, we hope these findings highlight the importance of infection control in any procedural skills, which should be regularly audited and focus upon in national guidance. Whilst practice is moving from transrectal to transperineal biopsy the potential role of environmental pathogens in post procedure infection and importance of incorporating good infection control procedures into local standard operating policies remain.
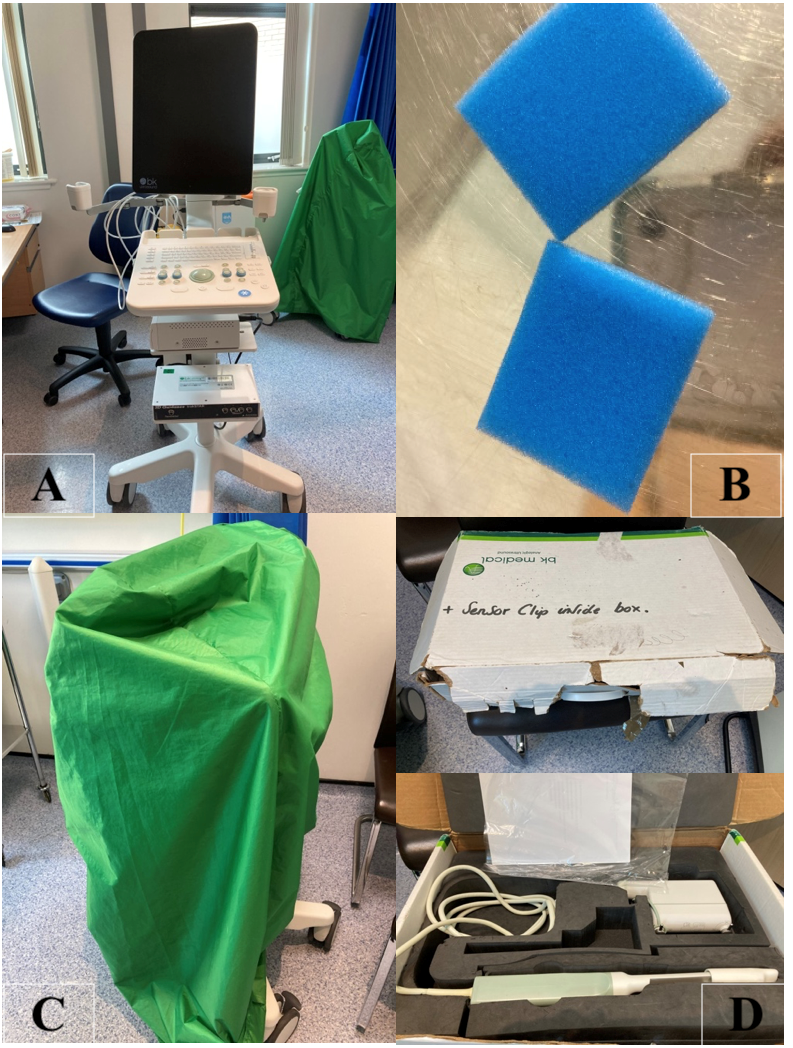
Figure 1
Conclusion
Here we have demonstrated the importance of infection control in conducting prostate biopsies, and reducing the rate of sepsis, especially when the offending bacteria is an environmental organism. The main interventions put into place were: to limit the number of people in the procedural room, to thoroughly clean the room and ultrasound machine in between each case, ensure room is well ventilated, provide online training to utilize Tristel cleaning agent and put in place a hand washing protocol. We hope other trusts follow our lead, and conduct audits in their departments to improve their rate of sepsis post TRUS/TP biopsies.
References
- Thomson A, Li M, Grummet J, Sengupta S. Transperineal prostate biopsy: a review of technique. Transl Androl Urol, 2020; 9(6): 3009-3017. doi: 10.21037/tau.2019.12.40.
- Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol, 1989; 142: 66–70.
- Wagenlehner FM, van Oostrum E, Tenke P, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol, 2013; 63: 521–527.
- Aron M, Rajeev TP, Gupta NP. Antibiotic prophylaxis for transrectal needle biopsy of the prostate: a randomized controlled study. BJU Int, 2000; 85: 682–685.
- The British Association of Urological Surgeons Limited. Transrectal ultrasound guided biopsies of the prostate gland, leaflet number 21/108, 2021.
- Bennett HY, et al. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect, 2016; 144: 1784.
- Sanders N, Buchan N. Infection-related hospital admissions after trans- rectal biopsy of the prostate. ANZ. J. Surg, 2013; 83: 246–248.
- Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol, 2011; 186: 1830–1834.
- Shahait M, Degheili J, El-Merhi F, Tamim H, Nasr R. Incidence of sepsis following transrectal ultrasound guided prostate biopsy at a tertiary-care medical center in Lebanon. Int Braz J Urol, 2016; 42: 60–68.
- Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol, 2013; 189(1 Suppl): S12–S17.
- Lei H, Dong X, Li L, Huan F, Zhong X, Wu Q, et al. Retrospective Study of the Etiology and Risk Factors of Systemic Inflammatory Response Syndrome After Systematic Transrectal Ultrasound-Guided Prostate Biopsy. Infect Drug Resist. 2020; 13: 3187-3193. doi: 10.2147/IDR.S256548.
- Raheem OA, Casey RG, Galvin DJ, Manecksha RP, Varadaraj H, McDermott T, et al. Discontinuation of anticoagulant or antiplatelet therapy for transrectal ultrasound-guided prostate biopsies: a single-center experience. Korean J Urol, 2012; 53(4): 234-239.
- Walker JT, Singla N, Roehrborn CG. Reducing Infectious Complications Following Transrectal Ultrasound-guided Prostate Biopsy: A Systematic Review. Rev Urol, 2016; 18(2): 73-89. doi: 10.3909/riu0713. PMID: 27601966
- Khanna A, Khanna M, Aggarwal A. Serratia marcescens- a rare opportunistic nosocomial pathogen and measures to limit its spread in hospitalized patients. J Clin Diagn Res, 2013; 7(2): 243-246. doi:10.7860/JCDR/2013/5010.2737
- EAU-ESUR-ESTRO-SIOG Guidelines on Prostate Cancer, 2019.
- National Institute for Health and Care Excellence (NICE). Prostate cancer: diagnosis and management NICE guideline (NG131), 2019.
- Lopez JF, Campbell A, Omer A, et al. Local anaesthetic transperineal (LATP) prostate biopsy using a probe-mounted transperineal access system: a multicentre prospective outcome analysis. BJU Int. 2021; 128(3): 311-318. doi: 10.1111/bju.15337.
- Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int, 2014; 114: 384–388. doi: 10.1111/bju.12536.
- Thompson PM, Pryr JP, Williams JP, et al. The problem of infection after prostatic biopsy: the case for the transperineal approach. Br. J. Urol, 1982; 54: 736–740.
- Werneburg GT, Adler A, Zhang A, et al. Transperineal Prostate Biopsy is Associated with Lower Tissue Core Pathogen Burden Relative to Transrectal Biopsy: Mechanistic Underpinnings for Lower Infection Risk in the Transperineal Approach. Urology. 2022; 165: 1-8. doi: 10.1016/j.urology.2022.04.013.
- Basourakos SP, Alshak MN, Lewicki PJ, et al. Role of Prophylactic Antibiotics in Transperineal Prostate Biopsy: A Systematic Review and Meta-analysis. Eur Urol Open Sci, 2022; 37: 53-63. doi: 10.1016/j.euros.2022.01.001.

