Hidradenitis Suppurativa: A Case Series and Review of Management Strategies with a Focus on Surgical Intervention
Shaniah Holder1,*, Marie Wisa Beauge2, De’jeau Pyfrom3, Muhammad Zain Ali3 and Frederick Tiesenga4
1Department of Medicine, American University of Barbados School of Medicine, Barbados
2Department of Medicine, Saint George’s School of Medicine, Grenada
3Department of Medicine, Saint James School of Medicine, Anguilla
4Department of Surgery, Community First Hospital, USA
Received Date: 26/01/2023; Published Date: 01/03/2023
*Corresponding author: Shaniah Holder, Department of Medicine, American University of Barbados School of Medicine, Barbados
Abstract
Hidradenitis Suppurativa (HS) is a chronic, relapsing inflammatory cutaneous disorder, characterized by involvement of the infundibular terminal follicles of skin folds in areas of rich apocrine glands. It presents as recurrent, fistulating, and draining sinuses with associated scarring and abscesses. Typical management through conservative approaches like smoking cessation, weight loss, wound care, and topical or oral medications can be helpful. However, treatment involves extensive surgical intervention when patients have multiple affected skin areas and recurrent or persistent disease. We present two patients diagnosed with HS and presented with unrelenting symptoms. Both patients showed drastic improvement following surgical intervention. Furthermore, we review the literature to explain the associated comorbidities, complications, and effective surgical repair methods for appearance restoration.
Keywords: Hidradenitis Suppurativa; Inflammatory Cutaneous Disorder; Intertriginous Areas; Surgical Management; Sharp Excision
Introduction
Hidradenitis Suppurativa (HS) is a chronic, debilitating inflammatory skin disease characterized by painful nodules and abscesses. These abscesses predominantly occur in the terminal hairs and apocrine sweat glands [1,2]. The risk factors for this disorder include poor lifestyle practices such as smoking, obesity, hormone imbalance, and the environment [3-5].
The estimated prevalence of HS ranges from one to four percent, predominantly in the African American population, with a higher incidence in women [7,8]. It is pivotal to know the three key elements of this disease to diagnose and treat patients in a timely fashion. They are (i) characteristic inflamed lesions, (ii) flexural site lesions, mainly the groin and axillae, and (iii) chronicity of presentation [3]. Medical therapies, including antibiotics, immunosuppressive drugs, and systemic retinoids, have helped ease symptoms. However, HS has a tendency to recur after initial treatment [5]. As the disease progresses, abscesses, odiferous draining sinus tracts, and irregular hypertrophic scarring can form, often declining the quality of life in patients [1,2]. Rarely, the ensuing chronic inflammation can lead to malignant transformation to squamous cell carcinoma, which is refractory to antibiotic or anti-inflammatory drugs necessitating surgical intervention [5]. Currently, a wide range of surgical treatments is available for the management of HS. However, symptomatic relief via local destruction or decompression of the lesions is more commonly used [2,5]. As this disease can be psychosocially draining on patients, it is essential to discuss interventions that could be curative.
Here, we present two patients diagnosed with severe HS that showed improvement following wide surgical excision of the lesion.
Case 1
A 32-year-old female with a medical history significant for Polycystic Ovarian Syndrome (PCOS) and Diabetes Mellitus II (DM II) presented for elective surgical excision of the right groin and thigh lesions caused by HS. She was diagnosed 7 months prior when she found “boils” on her thigh and groin region that grew and ulcerated leading to pain and discomfort when walking. The patient underwent wide excision and during the procedure, two separate abscesses with a total area of 10 x 10 centimeters (cm) were approached. The ulcers were sharply excised in an elliptical fashion with a large amount of pus encountered. (Figure 1) below show the wounds after the procedure.

Figure 1: Shows the right inner thigh and groin region with precise margins after the wide excision procedure.
Samples of the excised tissue were collected for pathology and culture. Slough, fibrinous exudate, and nonviable tissue were removed with a curette, and the patient’s wounds were irrigated and packed open with subsequent wound vacuum-assisted closure placement. The pathology report showed tissue with abscess and fistula tract formation, foreign body giant cell reaction, chronic inflammation, and fibrosis, which were compatible with the effects of HS. Microbiological studies of the wound revealed Candida albicans colonization due to a concomitant yeast infection. The patient was subsequently put on a seven-day regimen of fluconazole. Wound care was consulted and Aquacel Ag® (ConvaTec, Princeton, NJ, USA) antimicrobial dressing was placed. The patient was advised to follow up for further wound care after discharge. Two weeks later, there was adequate vascularization and healthy tissue growth was visualized. (Figure 2) below show the wounds 20 days after surgical excision.
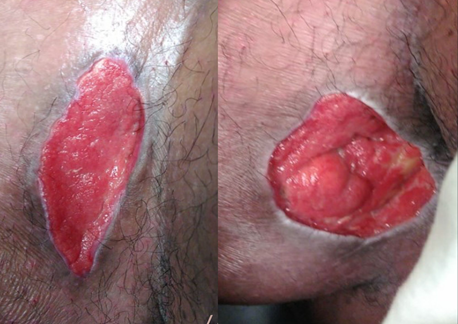
Figure 2: Shows the right groin wound 20 days after sharp wide excision of the lesion with normal tissue regeneration.
The patient showed signs of healing and was instructed to continue follow-up with wound care.
Case 2
A 56-year-old male with a 5-year history of HS presented to the ED with increased pain and drainage of a right gluteal wound despite ongoing wound care. Nine months prior to this hospital admission, he underwent surgical excision followed by antibiotic therapy, however, his symptoms recurred. On physical examination, the gluteal area was tender to light palpation with visualization of multiple subcutaneous inflammatory nodules and indentations. (Figure 3) represents the findings of the physical examination.
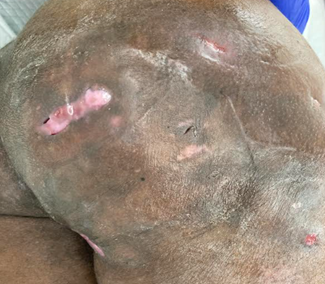
Figure 3: Shows fibrotic tissue with multiple site tracts.
The patient underwent wide excision and unroofing of the affected gluteal tissue which was approximately 20 x 40 cm. Multiple sinus tracts draining a large amount of pus were encountered. Meticulous hemostasis was assured and the patient did not have any postoperative complications apart from mild bleeding which was controlled. (Figure 4) shows images of the wounds on postoperative day two (POD 2) and postoperative day seven (POD 7).
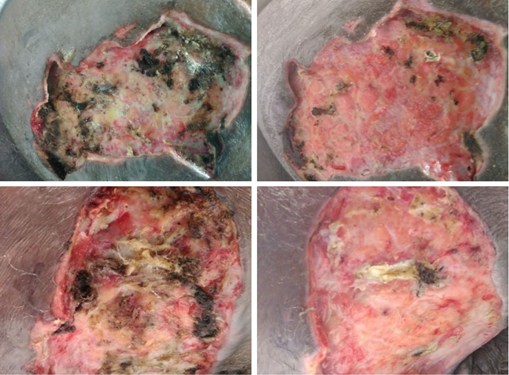
Figure 4: Shows wounds on POD-2 (left) and POD-7(right) with decreased scarring and tracts and increased granulation tissue visualized indicating healing.
The patient was discharged to a facility for wound care.
Discussion
The pathogenesis of HS includes epithelial hyperplasia of the ductal isthmus resulting in follicular hyperkeratosis, occlusion, and expansion. [9,10]. Friction and pressure on the skin cause molecular-sized antigen leakage, which stimulates the innate and adaptive immune system [1]. Consequently, cytokines are released which causes keratinocyte-induced activation of proinflammatory mediators [11,12]. If cellular damage is extensive then rupture of the follicular duct occurs precipitating the release of macro-follicular contents such as keratin fragments, sebum, and bacteria [1]. Prolonged response evolves into a chronic foreign body-type granulomatous inflammation [9].
HS presents as painful nodules in the intertriginous areas which progress to abscess formation and sinus tract development [2,8]. These sinus tracts become inflamed and drain seropurulent discharge. The late stage of HS presents with open or closed comedones and scarring after resolution which can range from fibrotic, rope-like bands to indurated, thick, scarred plaques causing disfigurement [1,10]. In this case, both patients had sinus tract formation and scarring. The diagnosis of HS is clinical and can often be misdiagnosed therefore a full skin examination is important [6].
HS is managed either conservatively, medically, or surgically and the Hurley staging system, which stratifies HS into three categories, is utilized [12]. Stage I is characterized by abscess formation only. Stage II is identified by recurrent abscesses with skin tunneling, scarring, and widely separated lesions. Stage III is marked by diffuse involvement of multiple connected skin tunnels and abscesses [1]. Lifestyle changes such as weight reduction, smoking cessation, and wound management are essential to implement. Antibiotics are the first-line medical therapy for Stage I or mild Stage II lesions and include topical agents such as Clindamycin [1]. Systemic agents like Clindamycin-Rifampicin and Rifampicin-Moxiflocacin-Metronidazole are required for severe lesions [6]. Finally, Adalimumab is effective in treating moderate to severe HS [12].
In severe, refractory HS, surgical intervention with punch debridement, unroofing, incision and drainage, or wide excision is indicated. Punch debridement involves the removal of the affected Follicular Pile Sebaceous Unit (FPSU) and its associated glands in one punch [11]. In a study where eleven patients received this procedure, only three had a recurrence, inferring adequate efficacy [11] Unroofing is beneficial for Stage III lesions and consists of the removal of the fibrotic skin with debridement of excess tissue, abscesses, and sinus tracts [12]. The wound is left open, allowing it to heal by secondary intention [6]. Incision and drainage is associated with a high disease recurrence rate since the underlying etiologies are not addressed [13]. Finally, wide excision is the ideal treatment of severe Stage II or III lesions and involves the removal of the entire affected area with margins beyond the clinical borders [1,12]. It is associated with longer healing times and a longer period of remission [14].
In a cohort study, 27 patients with a 7-year history of moderate to severe HS in multiple regions were treated by surgical excision [8]. Most patients were previously treated with non-surgical methods such as short-term antibiotic therapy and local wound care. 19 months after surgical intervention with wide excision coupled with graft placement, there were only two reports of recurrence [8]. Another study included 102 patients with Hurley stages I (13.5%), II (53.1%), and III (33.3%) HS who were treated with systemic antibiotics (88.2%), surgery (67.6%), antibiotic topical therapies (55.9%), and biologics (11.8%). The study reported that while antibiotics were more commonly used, surgery was the most effective treatment strategy [15]. These studies infer those conservative methods of treatment have a negligible effect on moderate to severe HS and surgical methods, specifically wide excision is preferred.
The patients in our study had Stage II and III HS and were managed with wide excision. After surgery, both patients reported improvement in their pain and their wounds showed considerable granulation tissue formation on follow-up. HS should be considered in any person with intertriginous nodules, skin tunnels, or abscesses and positive risk factors. Wide excision is the gold standard surgical procedure and is associated with decreased recurrence rates. It allows normal tissue regeneration and improves patients’ quality of life.
Conclusion
Urgent and thorough treatment methods are required for HS. Surgical intervention is associated with a higher rate of disease remission when compared to nonoperative methods. Specifically, the use of wide excision in our patients promoted pain reduction and immense granulation tissue formation while decreasing the risk of scarring, contracture formation, and malignant transformation. Additionally, patient education regarding lifestyle and continued wound care is crucial along with providing psychosocial support to patients combatting HS.
Author Contributions
Shaniah Holder: Concept and Design of study, Acquisition of data, drafting article, revising article. Guarantor Author
Marie Wisa Bejeau: Concept and Design of study, drafting article intellectual content, revising article
De’jeau Pyfrom: Acquisition of data, drafting article, revising article
Muhammad Zain Ali: Acquisition of data, drafting article, revising article
Frederick Tiesenga: Final Approval, Intellectual Content, revising article
Informed Consent: A signed statement of informed consent was obtained for publication of the details of this case
Competing Interests: None
Grant Information: None
References
- Napolitano M, Megna M, Timoshchuk E, Patruno C, Balato N, Fabbrocini G, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. CCID, 2017; 10: 105–115. DOI: 10.2147/ccid.s111019.
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Experimental Dermatology. 2008; 17: 455–472. DOI: 10.1111/j.1600-0625.2008.00712.x
- Ingram JR: The epidemiology of hidradenitis suppurativa*. Br. J. Dermatol, 2020; 183: 990–998. DOI: 10.1111/bjd.19435.
- Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers, 2020; 6. DOI: 10.1038/s41572-020-0149-1.
- Slade DEM, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. British Journal of Plastic Surgery. 2003; 56: 451–461. DOI: 10.1016/s0007-1226(03)00177-2.
- Ingram JR. Hidradenitis suppurativa: Pathogenesis, clinical features, and diagnosis. Uptodate, 2022.
- Jemec GBE: Hidradenitis Suppurativa. N Engl J Med, 2012; 366: 158–164. DOI: 10.1056/nejmcp1014163.
- Menderes A, Sunay O, Vayvada H, Yilmaz M. Surgical Management of Hidradenitis Suppurativa. Int. J. Med. Sci, 2010; 240–247. DOI: 10.7150/ijms.7.240.
- von Laffert M, Helmbold P, Wohlrab J, Fiedler E, Stadie V, Marsch WC. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol, 2010; 19(6): 533-537. DOI: 10.1111/j.1600-0625.2009.00915.x.
- von Laffert M, Stadie V, Wohlrab J, Marsch WC. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol, 2011; 164(2): 367-371. DOI: 10.1111/j.1365-2133.2010.10034.x.
- Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol, 2019; 81(1): 76-90. DOI: 10.1016/j.jaad.2019.02.067.
- Scuderi N, Monfrecola A, Dessy LA, Fabbrocini G, Megna M, Monfrecola G. Medical and Surgical Treatment of Hidradenitis Suppurativa: A Review. Skin Appendage Disord, 2017; 3(2): 95–110. DOI: 10.1159/000462979
- Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg, 2012; 38(4): 517-536. DOI: 10.1111/j.1524-4725.2011.02186.x.
- Kohorst JJ, Baum CL, Otley CC, Roenigk RK, Pemberton JH, Dozois EJ, et al. Patient Satisfaction and Quality of Life Following Surgery for Hidradenitis Suppurativa. Dermatol Surg, 2017; 43(1): 125-133. DOI: 10.1097/DSS.0000000000000942.
- Seyed Jafari S, Knüsel EM, Cazzaniga S, Hunger R. A Retrospective Cohort Study on Patients with Hidradenitis Suppurativa. Dermatology, 2018; 71-78. DOI: 10.1159/000488344.

