Nabiximols - An alternative to Gabapentin for Neuropathic Pain
Aditya Rai1,*, Naira Sargsyan2 and DN Prasad3
1Medico/Intern, Department of General Medicine, Sri Lakshmi Narayana Institute of Medical Sciences, India
2Physician, Department of Gastroenterology, Yeravan State Medical University after Mkhitar Heratsi, Armenia
3Professor, Department of Pharmacology, Shivalik College of Pharmacy, India
Received Date: 16/11/2022; Published Date: 30/01/2023
*Corresponding author: Aditya Rai, Medico/Intern, Department of General Medicine, Sri Lakshmi Narayana Institute of Medical Sciences, India
Abstract
Nabiximols also known as Sativex is a cannabis extract which was developed by GW Pharmaceuticals and approved in 2010 in the United Kingdom [1]. It is available as a mouth spray preparation [2] and is intended to treat neuropathic pain, overactive bladder, and spasticity along with certain symptoms of multiple sclerosis. Neuropathic pain is commonly caused due to disorders in the PNS or CNS. It is caused due to spinal cord injury [3], multiple sclerosis [4], and cerebrovascular accidents [5] in case of Central neuropathic pain and due to diabetes, herpes zoster infection, metabolic disorders, nutritional deficiencies, HIV-related neuropathies, toxins, remote manifestations of malignancies, immune mediated disorders and physical trauma to a nerve trunk [6] in case of Perpheral neuropathic pain.
Keywords: Nabiximols; Gabapentin; Neuropathic pain; Cannabinoids; THC; CBD; Sativex
Introduction
Neuropathic pain is most commonly diagnosed clinically and is typically characterized by pain with a sharp stabbing character with features like allodynia. It affects specific dermatomes which causes it to be limited to a certain area without spilling over. To find the exact pathology responsible for neuropathic pain, investigations have to be ordered for the most probable medical conditions including Quantitative Sensory Testing (QST) which gives a detailed analysis of the somatosensory system and can help in determining the subtype of neuropathic pain along with the best treatment modality.
The most commonly prescribed medications for neuropathic pain are Tricyclic antidepressants and Serotonin-norepinephrine reuptake inhibitors [7] or anticonvulsants like Pregabalin and Gabapentin [8]. As per various medical authorities, Gabapentin is recommended as a first line therapy for chronic neuropathic pain [9,10] and is used as a primary intervention in clinically diagnosed neuropathic pain. The exact mechanism of action of Gabapentin is not completely understood however since it affects voltage dependent calcium channels which can interrupt the events leading to dulling the neuropathic pain [11].
Nabiximols is a combination drug consisting of two principal active cannabinoid components - Tetrahydrocannabinol (THC) and Cannabidiol (CBD). The dose of each spray is 2.7 mg THC and 2.5 mg CBD. It is not FDA approved as of August 2022. RELEASE MSS1, a clinical trial to evaluate the effectiveness of Nabiximols for Multiple Sclerosis spasticity was carried out and future trials namely RELEASE MSS3 and RELEASE MSS5 are planned with an estimated 446 and 190 participants respectively to support a US FDA New Drug Application submission [12]. It has been approved to alleviate symptoms of multiple sclerosis, muscle spasms and neuropathic pain in about 25 countries including the Czech Republic, Denmark, Germany, Sweden, Italy, Austria, Poland, Canada and the UK.
Mechanism of Action
The mechanism of action of Nabiximols (Figure 1) is explained by understanding the endocannabinoid system (eCS). This consists of G protein-coupled cannabinoid receptors CB1R and CB2R along with endocannabinoid membrane transporter (EMT). The other important part of the mechanism includes the endocannabinoids namely anandamide (AEA) and 2-arachidonoylglycerol (2-AG) and the enzymes responsible for their breakdown for example monoacylglycerol lipase (MAGL) and fatty acid amino hydrolase (FAAH). The system is in use in numerous locations mainly associated with pain processing like the Dorsal root ganglion, thalamus, amygdala and the spinal cord [13].
CB1R modulation leads to the activation of the signalling cascade which inhibits Ca2+ intracellular influx that decreases fusion of the intracellular vesicles with the neuronal membrane. Linked to the microglia, CB2R expression is higher than that of CB1R and leads to the production and release of various cytokines [13].
In case of neuropathic pain, CB1R and CB2R expression is higher and enzymes like Fatty acid amide hydrolase (FAAH), Cyclooxygenase-2 (COX-2) and Arachidonate 5-lipoxygenase (5-LOX) increase their expression further. As an end result, AEA starts depleting and the pro-inflammatory mediators start increasing [13].
The above-mentioned mechanisms make the endocannabinoid system a very ideal target to inhibit neuropathic pain. THC acts as a CB1R and CB2R agonist which inhibits release of neurotransmitters by the by the neurons particularly glutamate and on top of this it also inhibits COX-2 which leads to increased levels of AEA and reduced levels of Prostaglandins thereby reducing pro-inflammatory signalling [13].
CBD is responsible for inhibiting eCB-degradation enzymes like FAAH and MAGL which in turn increases the level of AEA. CBD also acts on pain processing receptors in the neurons namely the glycinergic and serotoninergic receptors. A combination of CBD and THC results in synergistic effect in addition to reducing THC’s psychoactive side-effects like dysphoria, anxiety and panic attacks [13].
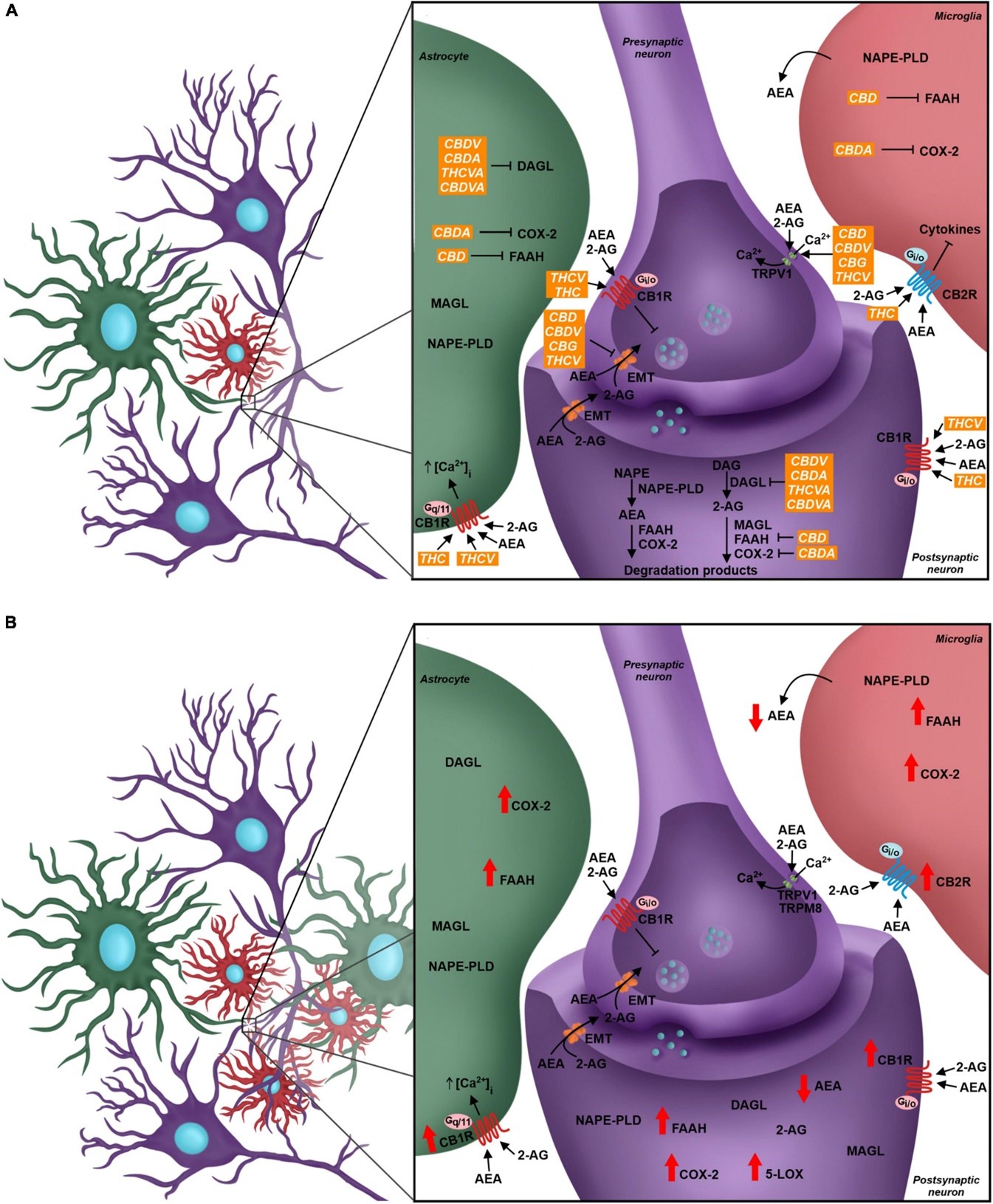
Figure 1: Diagrammatic representation of the endocannabinoid system in a quadripartite synapse in the event of neuropathic pain [13]. (A) All components of the endocannabinoid system with precursors, targets, inducers, inhibitors and end products. (B) Activity of all components as seen in a case of neuropathic pain.
Meta-analysis of Randomized Placebo-Controlled Trials
A meta-analysis was carried out to compile the results of 9 randomized placebo-controlled trials. These nine RCTs consisted of a total of 1,289 participants. The meta-analysis showed that with a small effect size, Nabiximols could reduce chronic neuropathic pain much significantly more than the placebo. The pooled endpoint of the mean difference in the fixed effect model was -0.40 and in the random effects model it was -0.44 both with a 95% confidence interval (Figure 2) [14].
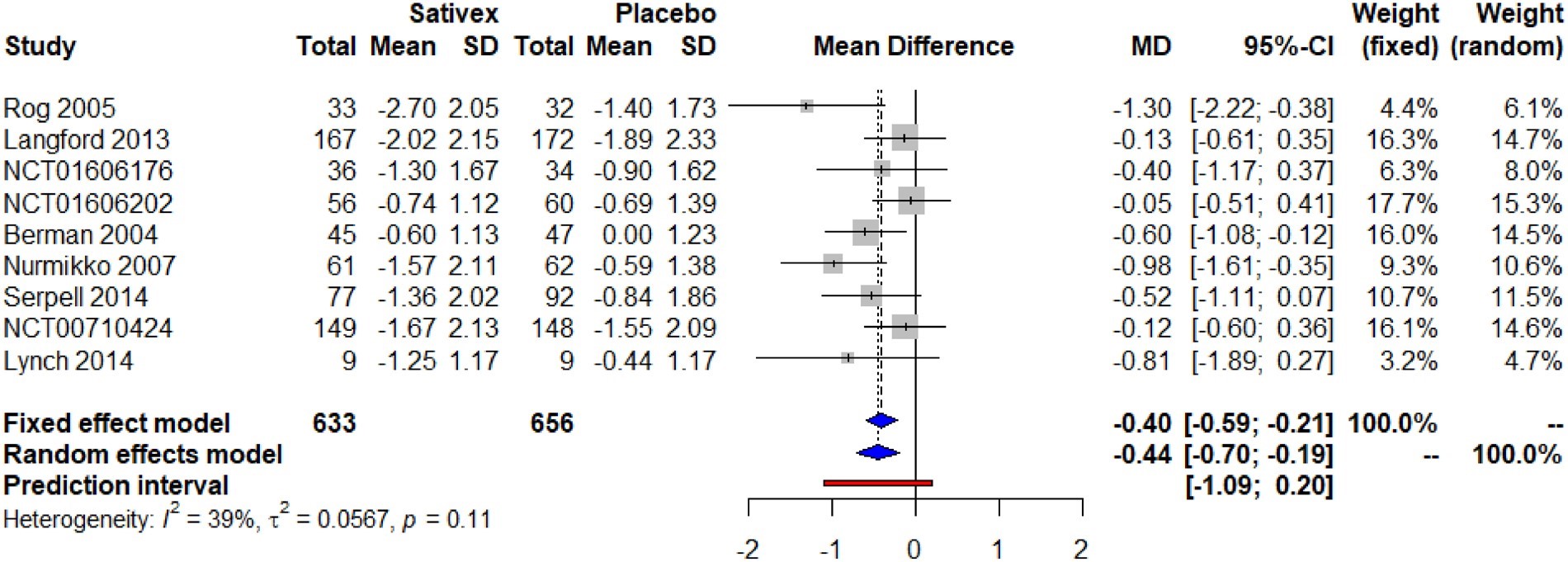
Figure 2: Change in pain scores with Nabiximols versus Placebo showing pooled endpoint of mean difference [14].
This is not conclusive however and much larger RCTs and their meta-analyses are required to provide more definitive answers [14]. This is with respect to other trials carried out for example a meta-analysis carried out for the use of opioids in chronic non-cancer pain which consisted of a cumulative 96 RCTs consisting of 26,169 participants out of which 25 RCTs were specifically for chronic neuropathic pain [15]. This was a lot larger than any similar study done for the use of Nabiximols in patients with chronic neuropathic pain.
Analgesic Effectiveness of Gabapentin as Compared to Nabiximols
Gabapentin when prescribed as 1800 mg or 3600 mg daily (1200 mg to 3600 mg gabapentin encarbil) is capable of providing pain relief to people with peripheral diabetic neuropathy and postherpetic neuralgia. A study carried out by the Cochrane Database of Systematic Reviews showed that 3-4 participants out of 10 participants for Gabapentin achieved at least 50% pain intensity reduction as compared to 1-2 participants out of 10 participants for placebo [16].
In a double blinded, placebo-controlled crossover pilot trial to evaluate the use of Nabiximols in chemotherapy-induced neuropathic pain, the number needed to treat for various analgesics was compared. Here, the NNT was 2.1 for Tricyclic antidepressants, 2.6 for opioids, 6.4 for gabapentin, 4.5 for pregabalin and 5.0 for Serotonin norepinephrine reuptake inhibitors. Nabiximols showed a NNT of 5.0 in a small pilot trial which is in a similar range to the other commonly used treatment modalities for neuropathic pain. This supports the theory that Nabiximols should be started as an adjuvant in cases of chemotherapy-induced neuropathic pain [17].
In addition to the above stated uses, Nabiximols have proven to be effective in patients with advanced cancer who have a poor response to opioid therapy. In a randomized controlled trial with a total of 360 randomized; 263 completed patients, the conclusion showed that Nabiximols were an effective and safe add-on analgesic for patients with opioid refractory cancer pain [18].
Discussion
Nabiximols has proven to be a very useful drug in almost all the trials that it has undergone. Even though it is approved in around 25 countries to treat chronic neuropathic pain, multiple sclerosis and cancer pain, it is still not approved by the FDA. Currently, the FDA has approved similar drugs for example - Epidiolex (cannabidiol), Marinol (dronabinol), Syndros (dronabinol), and Cesamet (nabilone) [19]. 39 states and Washington DC have legalized medical marijuana which suggests that the scope of using CBD and THC preparations has greatly improved in the past few years. Cannabis preparations are also a good substitute for dangerous prescription medications [20].
Nabiximols are safe and efficacious in treating chronic neuropathic pain, cancer associated neuropathic pain, symptoms of multiple sclerosis and opioid refractory cancer pain. While Gabapentin is a very effective drug for neuropathic pain, there are various scenarios in which Nabiximols would be well suited. It needs to be said that studies of a much larger magnitude are necessary to get more concrete data regarding its usage in a clinical setting to validate its effectiveness in treating chronic neuropathic pain and to support the FDA New Drug Application submission for Nabiximols. If the FDA approves Nabiximols, patients and clinicians alike will have more options on how to best manage chronic neuropathic pain.
References
- Multiple Sclerosis Trust. Sativex (nabiximols), 2014.
- Itin Constantin, Domb Abraham, Hoffman Amnon. "A meta-opinion: cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: the influences of fed / fasting states". Expert Opin Drug Deliv, 2019; 16(10): 1031–1035. doi:10.1080/17425247.2019.1653852.
- Kaur Jaskirat, Ghosh Shampa, Sahani Asish Kumar, Sinha Jitendra Kumar. "Mental Imagery as a Rehabilitative Therapy for Neuropathic Pain in People with Spinal Cord Injury: A Randomized Controlled Trial". Neurorehabilitation and Neural Repair, 2020; 34(11): 1038–1049. doi:10.1177/1545968320962498.
- Foley P, Vesterinen H, Laird B, et al. "Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta-analysis". Pain. 2013; 154 (5): 632–642. doi:10.1016/j.pain.2012.12.002.
- Baron Ralf, Binder Andreas, Wasner Gunnar. "Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment". The Lancet. Neurology, 2010; 9(8): 807–819. doi:10.1016/S1474-4422(10)70143-5.
- Vaillancourt PD, Langevin HM. "Painful peripheral neuropathies". Med. Clin. North Am, 1999; 83(3): 627–642. vi. doi:10.1016/S0025-7125(05)70127-9.
- Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. "Recommendations for the pharmacological management of neuropathic pain: an overview and literature update". Mayo Clinic Proceedings, 2010; 85(3 Suppl): S3-S14. doi:10.4065/mcp.2009.0649.
- Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. "Gabapentin for chronic neuropathic pain in adults". The Cochrane Database of Systematic Reviews, 2017; 6(2): CD007938. doi:10.1002/14651858.CD007938.pub4.
- Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. "EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision". European Journal of Neurology, 2010; 17(9): e1113–e1188. doi:10.1111/j.1468-1331.2010.02999.x.
- Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. "Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society". Pain Res Manag, 2007; 12(1): 13–21. doi:10.1155/2007/730785.
- Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. https://doi.org/10.1046/j.0003-2409.2001.02399.x.
- Jazz Pharmaceuticals Announces Top-line Results from Phase 3 Trial Evaluating Nabiximols Oromucosal Spray in Adult Participants with Multiple Sclerosis Spasticity.
- Campos RMP, Aguiar AFL, Paes-Colli Y, Trindade PMP, Ferreira BK, de Melo Reis RA, et al. Cannabinoid Therapeutics in Chronic Neuropathic Pain: From Animal Research to Human Treatment. Physiol. 2021; 12: 785176. doi: 10.3389/fphys.2021.785176.
- Igor Dykukha, Rolf Malessa, Ute Essner, Michael A Überall. Nabiximols in Chronic Neuropathic Pain: A Meta-Analysis of Randomized Placebo-Controlled Trials, Pain Medicine, 2021; 22(4): 861–874. https://doi.org/10.1093/pm/pnab050.
- Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA, 2018; 320(23): 2448-2460. doi: 10.1001/jama.2018.18472. PMID: 30561481; PMCID: PMC6583638.
- Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. "Gabapentin for chronic neuropathic pain in adults". The Cochrane Database of Systematic Reviews, 2017; 6(2): CD007938. doi:10.1002/14651858.CD007938.pub4. hdl:10044/1/52908. PMC 6452908. PMID 28597471.
- Lynch Mary E, Cesar-Rittenberg Paula, Hohmann Andrea G. "A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain". Journal of Pain and Symptom Management, 2014; 47(1): 166–173. doi:10.1016/j.jpainsymman.2013.02.018. ISSN 1873-6513. PMID 23742737.
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain, 2012; 13(5): 438-449. doi: 10.1016/j.jpain.2012.01.003. Epub 2012 Apr 5. PMID: 22483680.
- FDA and Cannabis: Research and Drug Approval Process.
- States With Medical Marijuana 2022.

