Successful Coronary Artery Bypass Surgery in a Patient with Myelodysplastic Syndrome
Hakkı Serkan Şahin1*, Hakan Çomaklı2, Özgür Altınbaş3, Tolga Soyal1 and Neyyir Tuncay Eren1
1Ankara Medicana International Hospital, Cardiovascular Surgery Clinic,Turkey
2Ankara City Hospital, Cardiovascular Surgery Clinic, Turkey
3Gaziantep University Vocational School of Health Services, Gaziantep, Turkey
Received Date: 29/11/2022; Published Date: 21/12/2022
*Corresponding author: Hakkı Serkan Şahin, Ankara Medicana International Hospital, Department of Cardiovascular Surgery/Sögütözü Caddesi Eskisehir Yolu Uzeri No:6, Post Code:06510 Cankaya/Ankara,Turkey
Abstract
Myelodysplastic Syndrome (MDS) is a heterogenous hematopoietic stem cell disease characterized by dysplastic and ineffective hematopoiesis. Hematopoietic system diseases need a multidisciplinary medical approach in the presence of cardiovascular diseases. A 63 year old male patient with MDS, recently diagnosed with multivessel coronary artery disease, underwent Coronary Artery Bypass Graft (CABG) surgery in our clinic and was discharged successfully on postoperative 10th day without any complication. In conclusion, the most probable complications following CABG in MDS patients are post-operative bleeding and infection, so careful perioperative evaluation and follow up can minimize these complications.
Keywords: Myelodysplastic Syndrome; Coronary Artery Bypass Surgery; Pancytopenia
Introduction
MDS consists of hematopoietic stem cell morphological dysplasia in the bone marrow, and peripheral cytopenia as result. The mean diagnosing age is 65, and the incidence increases with age and is higher in the male gender(1-4). The incidence over 60 age is 7-35/100.000 in the population(5). The main clinical expressions are commonly due to deep anemia, like exertional dyspnea or fatigue, and neutropenia like recurrent infections, and thrombocytopenia, like bleeding [1].
The International Study Group defined two requirements for the diagnosis of MDS. The first is cytopenia lasting over 6 months ( neutropenia < 1500/mm3, anemia Hb <11 g/dL, thrombocytopenia <100.000/mm3), and secondly the exclusion of clonal/non-clonal bone marrow diseases for cytopenia. Apart from the previous two primary criteria, one of the three sign must also coexist. These are; dysplasia ( existing over >10% in one or more bone marrow biopsies ), myeloblast ratio between 5-19%, the presence of MDS specific karyotypes ( like del(5q) and del(20q) ) [2].
Several prognostic systems exist in order to predict the progress of MDS. The commonly used one is The International Prognostic Scoring System (IPSS ). In time, with the understanding of the cytogenetic predictors’ importance in the prognosis, this system was revised to “Revised IPSS ( R-IPSS)”. These scoring systems rely on genetic identification, cytopenias, and myeloblast counts. MDS is classified as low or high risk accordingly to this scoring system [3,4].
In such patients, whenever an ischemic cardiac coronary event occurs, it’s the heart team who calls for the proper treatment, percutaneous intervention (PCI) or CABG. CABG is more favorable in such cases where due to thrombocytopenia, dual long term antiplatelet therapy is less desirable [5,6]. With a multidisciplinary strategic approach, a limit for the thrombocyte count can be set, for which method to follow, surgery or PCI.
Case Report
A 63-year-old male patient, following a chest and left arm pain starting 2 hours ago, was brought to the emergency room (ER), whose on the electrocardiogram had anterior derivation ST depression, from V1 to V4. Following nitrate infusion and 5000 IU heparin administration, the pain was improved.
The patient had a history of MDS diagnosed 3 years ago and was in the high-risk group. The patient, diagnosed with anterior MI, was taken into the Cath Lab. Coronary angiography showed proximal Left Anterior Descending Artery (LAD) 98%, 1sth diagonal( D ) 90 %, Intermediate artery (IMA) 70%, Obtuse Marginal (OM) 80%, Right Coronary Artery (RCA) mid 99%, Right originated Posterolateral Artery 80% lesions. As with such extended coronary lesions, CABG was the decision made by the heart team.
Due to the urgency of the coronary stenotic lesions, after admission to the cardiovascular clinic, the patient was consulted with the hematology department. There was no myeloblastic activation on the peripheral blood smear, leukemic progression risk was low enough to permit irradiated blood transfusion and rehabilitation for 4 to 6 weeks. The potential life expectancy benefit of the CABG over 1 year was overweighting the risks of MDS.
Left ventricular (LV) ejection fraction ( LVEF ) was 63% preoperatively, had mild LV anterior hypokinesis, and global LV hypertrophy. Laboratory findings were as follows; leucocyte count: 2.23 x103/ µL, neutrophil count: 0.58x103/L, hemoglobin: 7.6 g/dL, thrombocyte count: 49.3 x103/ µL, MCV: 112 fL, MCH: 39.3 pg, glucose:100mg/dl , BUN:28mg/dl, creatinine:1.05mg/dl, AST:15 U/L, ALT:16U/L, CRP:0.5 mg/dl, sedimentation 1h:60mm/h, troponin ( high sensitive ): <0.01 ng/ml, and CK-MB:0.2ng/dl.
The patient had an on pump 6 vessel CABG surgery ( LIMA-LAD, Ao-saphenous vein - OM1- OM2 – CxPL artery, Ao-saphenous vein - PDA - Right PL artery sequential anastomosis). Overall cardiopulmonary bypass ( CPB ) and cross clamp time was 81 minutes and 69 minutes, respectively.
The goal was to keep the early postoperative thrombocyte count over 50x103/µL so 1 unit of irradiated apheresis thrombocyte was given intravenously before surgery, and 1 following the termination of the CPB, with 1 fresh frozen plasma and 1 unit of an erythrocyte. In the ICU, again 2 units of irradiated apheresis thrombocyte were infused and iv. cefazolin 4x1 g and amikacin 1x1 g iv were administered for prophylaxis daily. The patient was disconnected from the mechanical ventilator on the 8th hour, mobilized on the 12th hour, and discharged from the ICU to the clinic on the 18th hour postoperatively. Total drainage was 800 cc on the 24th hour from the chest tubes. The chest tubes and the urinary catheter were removed on the 48th hour as no significant drainage occurred. On the blood count postoperative 1. day, as the leukocyte count was 0.9x103/ µL, Granulocyte Colony-Stimulating Factor(G-CSF) filgrastim ( Neupogen Roche 48 MU/0.5 ml ) 30 million units was injected subcutaneously. On the postop 2nd day hemogram leukocyte count was 1.49x103/ µL, 3rd day 2.15 x103/ µL, 4th day 2.13 x103/ µL and on the 5th day 1.95 x103/ µL. On the 5th day, a booster dose of 30 million units filgrastim was reinjected subcutaneously (Figure 1). On the 6th-day haemogram leukocyte count was 4.52x103/µL.
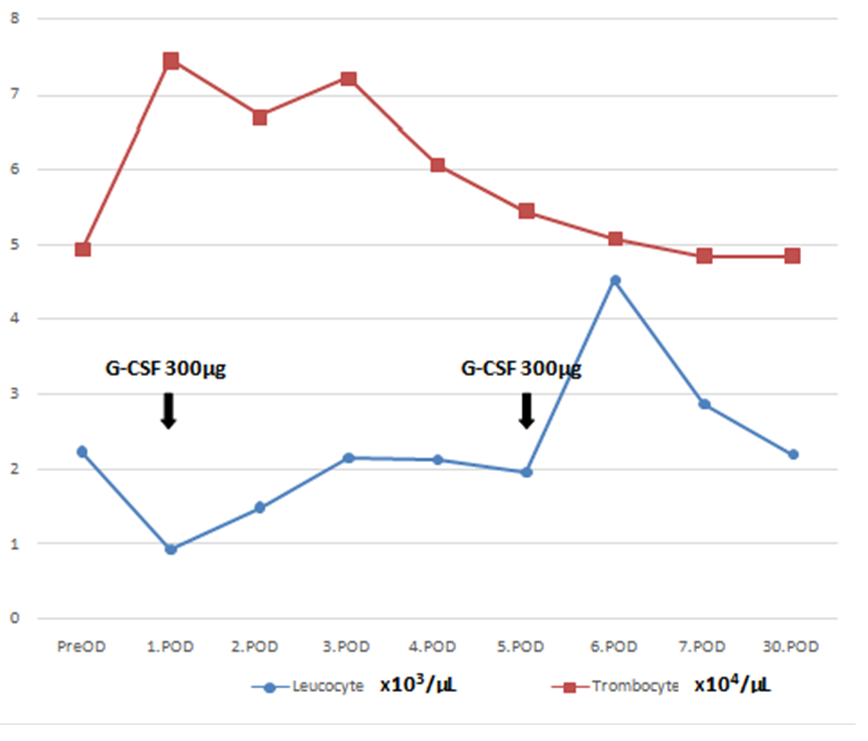
Figure 1: Daily Leucocyte and Trombocyte Counts. G-CSF; granulocyte colony-stimulating factor; PreOP; preoperative day. POD; postoperative day.
(Corrected trombocyte unit value to ensure Scale Compliance)
On the postoperative 4th day the patient suffered from a subfebrile fever of 37.5 ͦC, blood, urine and throat samples were taken for culture, where no microorganism was detected later on. The patient was discharged home on the hospital day 10 morning with 150 mg of daily acetylsalicylic acid orally. 7 days after hospital discharge a haemogram was done, leukocyte count was 2.87x103/ µL, and 2.20 x103 on the 30th day. The patient did not show any symptoms of infection on both physical exams.
The patient is in good condition in its second month of follow up, no sign of infection or hemorrhagic disorder yet by the time this case was reported.
Conclusion
Since MDS consists of hematopoietic stem cell morphological dysplasia in the bone marrow, and cytopenia, the mainstay of the perioperative treatment should focus on the replacement of the lacking cellular component. We used granulocyte colony-stimulating factor (G-CSF), irradiated red blood cells (RBCs) and platelet transfusions and antibiotic prophylaxis in the perioperative treatment. Our patient had no history of previous transfusion, where patients with MDS may need regular transfusions of RBCs or platelets in order to keep the hematopoietic screen at one level, and eventually develop anti platelet antibodies for a platelet specific antigen or HLA antigen and require HLA-matched platelets, which can complicate the perioperative preparations. G-CSF is a well known and reliable agent in promoting the bone marrow, where also has been used for such pancytopenic patients undergoing cardiac surgery [11,14].
Anemia, leucopenia, thrombocytopenia or thrombocytosis can be seen on the blood count. Pancytopenia can be seen in almost 50% of patients. Clinical manifestation can either be with chronic asymptomatic cytopenias or with symptomatic anemias, resulting in recurrent infections, bleeding or conversion to acute leukemia. Chemotherapies, mutant effects of radiotherapy over stem cells or genetic disorders are frequently addressed for the etiology of MDS [2,7,8]. Whatever the etiology may be, there are reports assessing thrombocytopenia as a poor prognostic factor for the ischemic heart diseases, as hemorrhagic risks limit the use of antiaggregant therapies. Russo et.al. admitted the benefit of administering dual antiplatelet therapy to a group of 9 in 13 of idiopathic thrombocytopenic purpura patients with ischemic heart disease [9].
Porta et al. in a cohort of 840 MDS patients, reported the presence of cardiac disease in 25% of the patients, and was the most common comorbidity and the leading cause of non-leukemic death. Age and comorbidity was found to be closely related, ranging from %29 in patients under the age of 50, while rising to %71 over the age of 75. The most frequent non-leukemic death causes were cardiac failure (63%), infection (23%), hemorrhage (7%) and hepatic failure ( 4% ) [12].
Sorror et al. reported cardiac comorbidities in patients with MDS before allogeneic hematopoietic cell transplantation; stating an overall cardiac comorbidity of %36 at the initial diagnosis (atrial fibrillation/flutter, sick sinus syndrome or ventricular arrhythmia like arrhythmias 7%, heart valve diseases %2, coronary artery disease or myocardial infarction like one or more vessel-coronary artery stenosis requiring medical treatment, stent or bypass graft 8%, ejection fraction below 50% or congestive heart failure 19%.) Transient ischemic attack and/or ischemic or hemorrhagic cerebrovascular accident presence was 5%. Mild (FEV1 66%-80%) pulmonary dysfunction presence was %3, severe (FEV1 <65%, dyspnea at rest or requiring oxygen) pulmonary dysfunction 2%. Mild or severe hepatic failure presence were 14% and 3%. Any renal failure with serum creatinine > 2mg/dL was 4%. A solid malignant tumor presence was 10%, any rheumatological condition; rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, mixed connective tissue disease presence were 2%, any gastrointestinal condition like ulcerative colitis,Crohn’s disease or peptic ulcer requiring treatment presence were 6%, Diabetes mellitus presence was %11, major endocrine disorders like thyroid disorders, parathyroid gland disorders, adrenal disorders, pituitary gland disorders, or hypogonadism were 5%, obesity 2%, and psychiatric disorders were present in 2% patients [13].
Patients with MDS may undergo CABG safely unless each and every system is carefully evaluated perioperatively, presuming the potential existence of multiple comorbidities, where according to the urgency of the CABG, each medical condition can be treated accordingly. Zhang et al presented a successfully treated risky MDS patient with CABG in the ACC 2019 meeting [10]. Again Yamagashi et al reported a similar case in 1996 [11].
We recommend a multidisciplinary approach for these patients in deciding the most beneficial therapy as both MDS and cardiovascular diseases have life-threatening consequences.
References
- Abdel-Wahab O, Figueroa ME. Interpreting new molecular genetics in myelodysplastic syndromes. ASH Education Program Book, 2012: 56-64.
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia, 2008; 22: 14–22.
- Myelodysplastic Syndromes. NCCN Practice Guidelines in Oncology, 2016; 1.
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F. et al. Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood, 2012; 120: 2454-2465.
- Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA. et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood, 2008; 112(1): 45-52.
- Morici N, Cantoni S, Savonitto S. Antiplatelet therapy for patients with stable ischemic heart disease and baseline thrombocytopenia: Ask the hematologist. Platelets, 2014; 25: 455–460.
- Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of hematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. British Journal of Cancer, 2011; 105(11): 1684-1692.
- Kuendgen A, Strupp C, Aivado M, Hildebrandt B, Haas R, Gattermann N, et al. Myelodysplastic syndromes in patients younger than age 50. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 2006; 24(34): 5358-5365.
- Russo A, Cannizzo M, Ghetti G, Barbaresi E, Filippini E, Specchia S, et al. Idiopathic thrombocytopenic purpura and coronary artery disease: Comparison between coronary artery bypass grafting and percutaneous coronary intervention. Interact CardioVasc Thorac Surg, 2011; 13: 153–157.
- Zhang K, Wiesehan H, Westervelt P, Maniar H, Krone R, Lenihan D. Coronary Artery Bypass Grafting in High-Risk Myelodysplastic Syndrome. J Am Coll Cardiol, 2019; 73: 2688-2691.
- Yamagishi T, Fuse K, Saito T, Kato M, Misawa Y, et al. Successful coronary artery bypass grafting for a patient with myelodysplastic syndrome: report of a case. Surg Today, 1996; 26(9): 740-743.
- Porta MGD, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica, 2011; 96(3): 441-449.
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT) specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood, 2005; 106(8): 2912-2919.
- Miyagi Y, Yamauchi S, Suzuki S, Kitagawa A, Masaki Y, et al. Investigation of coronary artery bypass grafting for a patient with myelodysplastic syndrome. Ann Thorac Cardiovasc Surg, 2001; 7(4): 250-253.

