Percutaneous Renal tumor biopsy: Is it a Friend or an Enemy?
Mahmoud Alafifi1,2,*, Yassine Larrache1,2, Yassine Daghdagh1,2, Amine Moataz1,2, Mohamed DAKIR1,2, Adil DEBBAGH1,2, Rachid ABOUTAIEB1,2
1Department of Urology, University Hospital Center Ibn Rochd Casablanca, Morocco
2Faculty of Medicine and Pharmacy of Casablanca Morocco
Received Date: 08/11/2022; Published Date: 25/11/2022
*Corresponding author: Mahmoud Alafifi, Department of Urology, University Hospital Center Ibn Rochd Casablanca, Morocco
Abstract
Percutaneous Renal Mass Biopsy (PRMB) has a long history of use in the treatment of radiologically indeterminate renal masses.
Surgery, ablative therapy, or active surveillance may be offered to patients with small renal masses who have biopsy-proven Renal Cell Carcinoma (RCC), and PRMB can provide diagnostic tissue from patients with metastatic disease who may benefit from systemic therapy.
PRMB is considered a safe procedure with extremely low complication rates, the vast majority of which are minor and resolve spontaneously. Seeding of renal tumors after biopsy is extremely rare.
The utility of the information gained from PRMB must be balanced against the small but significant risk of seeding when deciding on the best course of action for patients with renal masses.
We present a rare case of RCC seeding following PRMB and review of current literature.
Keywords: Renal cell carcinoma; Tumor seeding; Percutaneous renal mass biopsy; Complication
Introduction
A pathological diagnosis is the first step toward treatment for the vast majority of cancer patients.
In the case of kidney cancer, imaging has been accepted as a substitute for histology.
On the other hand, Percutaneous renal mass biopsy (PRMB) has a long history of use in the treatment of radiologically indeterminate renal masses.
PRMB has a number of advantages, including the identification of potentially cancerous cells and the development of an appropriate treatment plan based on the results.
Like any other procedure, PRMBs carry risks, the vast majority of which are minor and resolve spontaneously.
Seeding of renal tumors, a process in which malignant cells may be carried into normal tissues along the path of a needle, is one such risk that is generally regarded as theoretical due to an estimated occurrence rate of less than 0.01 percent [1].
We present a rare case of RCC seeding following PRMB and review of current literature.
Case Presentation
A 75-year-old woman with a history of right renal tumour which was suspected on abdominal CT (Figure 1) and confirmed by a percutaneous ultrasound guided renal biopsy with an 18-gauge Tru-Cut,
After further discussion at the multidisciplinary meeting, anti-angiogenic treatment with sunitinib for 2 months followed by a right enlarged total nephrectomy was recommended, after which the patient was lost to follow-up. The recurrence of the right masse (Figure 2) highlighted the evolution.
An abdominal CT scan confirmed the presence of a polylobed tissue mass with irregular contours at the level of the 11th right intercostal space, measuring approximately 48 x 39 mm extended over 36 mm; in front, this mass comes into contact with the posterior arch of the 11th rib, the cortical of which has a blurred and irregular appearance at this point; externally, it dislodges and appears to envelop in places the internal oblique, external oblique muscles, which are amyotrophic (Figure 3).
After further discussion at the multidisciplinary meeting, surgical resection of the right parietal mass was recommended (Figure 4).
The patient was discharged on day 4 following the surgical resection without complication. She was reviewed 2 weeks postoperatively to discuss pathology results. 4 months following surgical resection the patient is well.
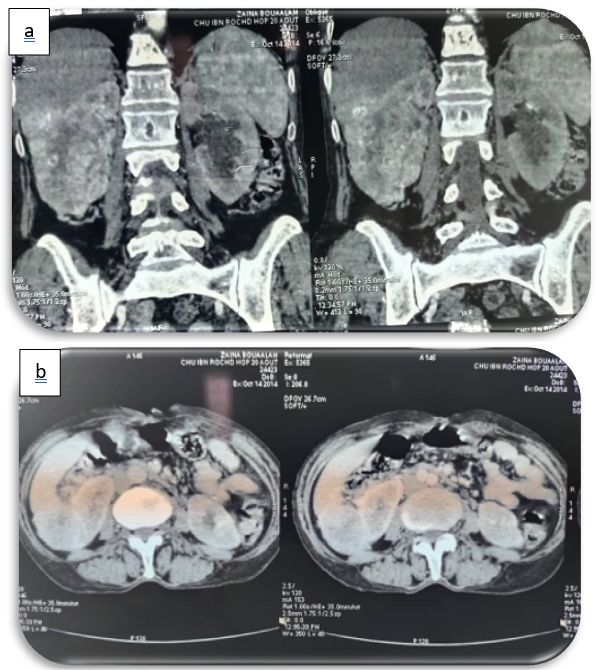
Figure 1a, 1b: coronal and axial sections, showing an infiltrating mass at the expense of the right kidney, with ill-defined contours, heterogeneously enhanced after PDC injection.

Figure 2: Right parietal mass.
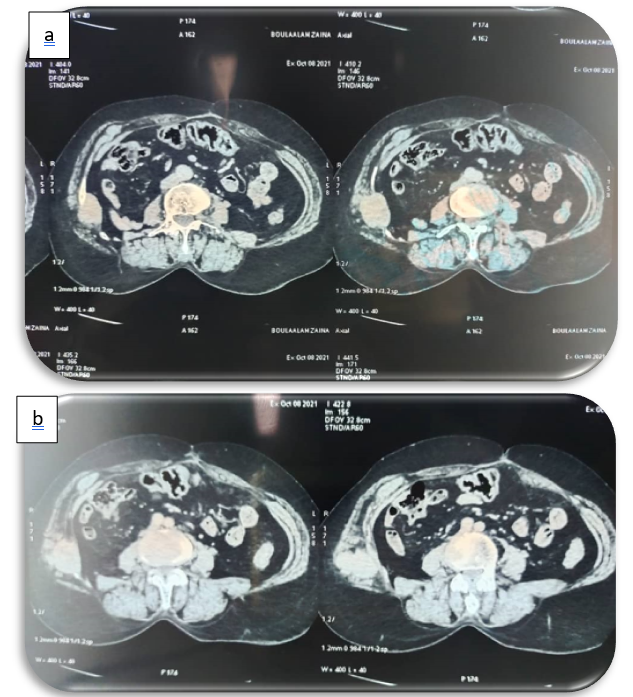
Figure 3a, 3b: axial sections showing a lytic mass opposite the nephrectomy scar, centred on the middle and posterior arches of the right 10th side, well limited with irregular contours, heterogeneously enhanced, delimiting a central zone of necrosis, and extended to the soft parts opposite.

Figure 4: Surgical specimen.
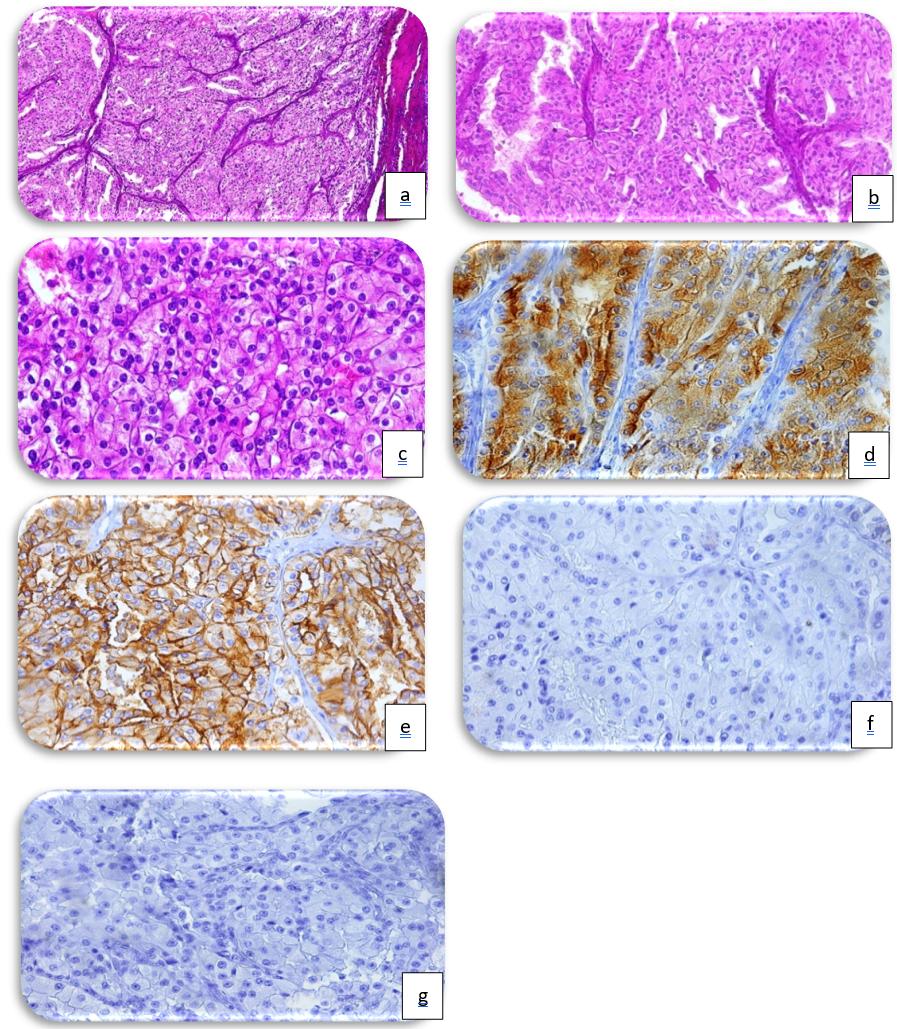
Histological sections confirmed that a carcinomatous growth with a solid, tubular architecture enclosed by a fibrous capsule can be detected (Figure 4a, HE, x10). The cells are cubic to cylindrical at medium magnification, having clear or eosinophilic cytoplasm inside a fibrovascular stroma (Figure 4b, HE, x20). The nuclei are oval, central, with unevenly scattered chromatin in coarse clusters and one to three nucleoli [nucleoli are only discernible at high magnification] (Figure 4c, HE, x40). The tumour cells express CCR (Figure 4d) and CD10 (Figure 4e) diffusely and strongly, but not CK7 (Figure 4f) or CD117 (Figure 4g).
Discussion
With rapid advances in medical imaging technology and treatment modalities, the indications of and demand for PRMBs have been expanding [2].
PRMB can be of value in characterizing renal masses in selected patients, [3,4] and may be helpful in triaging patients to an increasing assortment of therapeutic options. [5,6]
The American Urological Association (AUA) recommends that a PRMB be performed prior to ablation therapy to provide pathologic diagnosis and to guide subsequent surveillance [7]. Furthermore, in several systemic analyses [7–10], PRMB is considered highly accurate for the diagnosis of malignancy and histologic determination of RCC subtypes. PRMB is widely regarded as a safe procedure.
Other than hematoma, complications are rare. Tumor seeding along the needle tract, arteriovenous fistula formation, infection, and pneumothorax [11–13].
Needle track seeding in other organs has been described and reviewed [14-18], but is relatively uncommon in renal mass biopsy. Needle track seeding by tumor cells in renal mass biopsy is a complication that has been reported when larger (up to 14-gauge) needles often were used [19-22], but had become vanishingly rare.
This procedure employs a wide range of needle sizes, ranging from 21-gauge to 27-gauge needles for Fine-Needle Aspiration (FNA) to 17-gauge to 20-gauge needles for core needle biopsy. Core biopsy needles as large as 11 gauge are also available, but are rarely used in this location. FNA and core needle biopsies are both precise tests with sensitivities that can exceed 90% [4]. Core needle biopsy is generally more sensitive than FNA because it obtains more tissue, but the combination of FNA and core needle biopsy is reported to achieve the highest sensitivity [23-25].
The incidence is not well characterized, but in 1 large metaanalysis there was 1 case reported in nearly 3000 patients [4]. The vast majority of needle track cases were papillary renal cell carcinomas (14 of 16 cases; one clear cell tumor and one not specified), and the majority have been reported to be low-grade tumors.
Clearly, something about papillary tumors makes it much easier for them to seed the needle track than other types of renal cell carcinoma, some of which are much more common. Low-grade papillary tumors are known to be friable, which may explain this outcome in part because tumor cells may adhere to the outside of the needle and fall off as it is removed from the patient. Furthermore, low-grade papillary tumors are frequently exophytic tumors, so if any cells are tracked along the biopsy path, they will be in the extrarenal space rather than the kidney itself.
Surgeons also know that if they accidentally incise the capsule of some renal cell carcinomas, the lesion will ooze out of the incision like toothpaste under pressure, and papillary tumours may be more likely to do so. Finally, when compared to other types of tumours, papillary tumour cells may be more likely to survive when explanted into the needle track.
Is it possible to avoid needle track seeding? The CCAFU Kidney Group advises the use of a 16 to 18G (weak) Truc-cut needle positioned in a coaxial needle in order to take at least two specimens, to avoid necrotic territories and to limit the risk of needle track seeding [26].
Alternatively, if a papillary renal cell carcinoma is diagnosed on biopsy, the surgeon could excise the needle track when the lesion is resected. To date, all reported cases have only revealed tumor cells in the adipose tissue near the kidney or in the subcutaneous tissue near the needle site, necessitating the removal of both the skin and adipose tissue along the needle track [27].
Historically, renal biopsies were used to diagnose secondary, metastatic renal tumors as well as benign non-tumor pathologies such as renal abscess [28].
Many centers are now accepting that PRMB should be offered to most, if not all patients with small renal masses, including those who are potential candidates for surgery or ablative therapy (pre-treatment) as well as active surveillance [29, 30]Other indications include post-ablative therapy for suspected recurrence, confirmation of full ablation, and primary RCC subtype characterization in the presence of metastatic illness in order to identify the best biological systemic therapy (particularly when a cytoreductive nephrectomy is not indicated). The role of biopsy in larger localized tumors (> T1b) is debatable, but it may be used. The role of biopsy in larger localized tumors (> T1b) is debatable, but it may be used more frequently when partial nephrectomy is being considered to rule out high grade tumors with a theoretically higher risk of local recurrence, as well as the less common, large oncocytoma or fat-poor component angiomyolipoma. PRMB has only a few contraindications. The only absolute is irreversible coagulopathy. Patients with a limited life expectancy who are not candidates for any surgical, ablative, or medial treatment, since the outcomes would not change the management strategy, may be relative contraindications for PRMB.
The primary goal of a renal biopsy should be to determine the diagnosis, subtype grade, and molecular features of each tumour.
Tumor seeding following renal tumor biopsy in patients with RCC deserves more attention.
Future studies with a large sample size and longe follow-up are needed to determine the relationship between needle tract tumour seeding and prognosis.
Conclusion
To minimize the risk of seeding, needle size and technique used should be taken into consideration before a biopsy is performed.
The utility of the information gained from PRMB must be balanced against the small but significant risk of seeding when deciding on the best course of action for patients with renal masses.
Conflicts of Interest: No
References
- Caoili Elaine M, Davenport Matthew S. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol, 2014; 31: 20–26.
- Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol, 2011; 66(11): 1007–1014.
- Renshaw AA. Kidney and adrenal gland. In: Cibas ES, Ducatman BS, eds. Cytology: Diagnostic Principles and Clincal Correlates. 4th ed. Edinburgh: Saunders, 2014: 383-404.
- Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol, 2016; 69: 660-673.
- Sanchez A, Feldman AS, Hakimi AA. Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermal ablation, 2018. J Clin Oncol. doi:10.1200/JCO.2018.79.2341.
- Huang X, Griffin B, Lin X. Management of patients with renal mass lesions based on renal biopsy cytology results. Hum Pathol, 2019; 85: 270-278.
- Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol, 2017; 198(3): 520–529.
- Lebret T, Poulain JE, Molinie V, Herve JM, Denoux Y, Guth A, et al. Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol, 2007; 178(4 Pt 1): 1184–1188.
- Neuzillet Y, Lechevallier E, Andre M, Daniel L, Coulange C. Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. J Urol, 2004; 171(5): 1802–1805.
- Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol, 2016; 69(4): 660–673.
- Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, Saravanan A, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol, 2007; 178(2): 379–386.
- Busset C, Vijgen S, Lhermitte B, Yan P. A case report of papillary renal cell carcinoma seeding along a percutaneous biopsy tract. Open Journal of Pathology, 2018; 8: 139–146.
- Herts BR, Baker ME. The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol, 1995; 13(4): 254–261.
- Valle LG, Rocha RD, Mendes GF, Succi JE, de Andrade JR. Tumor seeding along the needle track after percutaneous lung biopsy [in English and Portuguese]. J Bras Pneumol, 2016; 42: 71.
- Shah KS, Ethunandan M. Tumour seeding after fine-needle aspiration and core biopsy of the head and neck–a systematic review. Br J Oral Maxillofac Surg, 2016; 54: 260-265.
- Tchatalbachev VV, Kirkpatrick DL, Duff DJ, Travis MD. Seeding of the rectus sheath with hepatocellular carcinoma after image guided percutaneous liver biopsy using coaxial biopsy needle system. J Radiol Case Rep, 2015; 9: 18-25.
- Lee CU, Kim SJ, Sung JY, Park SH, Chong S, Baek JH. Needle track tumor seeding after radiofrequency ablation of a thyroid tumor. Jpn J Radiol, 2014; 32: 661-663.
- Volanis D, Neal DE, Warren AY, Gnanapragasam VJ. Incidence of needle-tract seeding following prostate biopsy for suspected cancer: a review of the literature. BJU Int. 2015; 115: 698-704.
- Wehle MJ, Grabstald H. Contraindications to needle aspiration of a solid renal mass: tumor dissemination by renal needle aspiration. J Urol, 1986; 136: 446-448.
- Gibbons RP, Bush WH Jr, Burnett LL. Needle tract seeding following aspiration of renal cell carcinoma. J Urol, 1977; 118: 865-867.
- Shenoy PD, Lakhkar BN, Ghosh MK, Patil UD. Cutaneous seeding of renal carcinoma by Chiba needle aspiration biopsy. Case report. Acta Radiol, 1991; 32: 50-52.
- Kiser GC, Totonchy M, Barry JM. Needle tract seeding after percutaneous renal adenocarcinoma aspiration. J Urol, 1986; 136: 1292-1293.
- Li G, Cuilleron M, Zhao A, et al. Combination of core biopsy and fine-needle aspiration increases diagnostic rate for small solid renal tumors. Anticancer Res, 2012; 32: 3463-3466.
- Cate F, Kapp ME, Arnold SA, et al. Core needle biopsy and fine needle aspiration alone or in combination: diagnostic accuracy and impact on management of renal masses. J Urol, 2017; 197: 1396-1402.
- Tsivian M, Rampersaud EN Jr, del Pilar Laguna Pes M, et al. Small renal mass biopsy–how, what and when: report from an international consensus panel. BJU Int, 2014; 113: 854-863.
- Bensalah K, Albiges L, Bernhard J -C, Bigot P, Bodin T, Boissier R, et al. French Recommendations of the Afu Cancer Committee Update 2018–2020: Management of Kidney Cancer. Méjean Reference: Prog Urol, 2018, 28(Sup 1): R5.
- Andrew A Renshaw, Alex Powell, Jorge Caso, Edwin W Gould. Needle Track Seeding in Renal Mass Biopsies.
- Herts BR, Baker ME. The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol, 1995; 13: 254-261.
- Rendon RA, Kapoor A, Breau R, et al. Surgical management of renal cell carcinoma: Canadian Kidney Cancer Forum Consensus. Can Urol Assoc J, 2014; 8: E398-412.
- Jewett MA, Finelli A. Kidney cancer: Routine small renal mass needle biopsy should be adopted. Nat Rev Urol, 2014; 11: 548-549.
- Alafifi M, Mostapha A, Dibingue C, AlAfifi R, Karam R, Marnissi F, et al. Metachronous Giant Renal Angiomyolipoma: About One Case. Am J Urol Res, 2022; 7(1): 001-004.
- Alafifi mahmoud, Elkebir A. Renal Tumors: Risk Factors, Clinical Profile, and Histopronosis: A Review of 58 Cases. Journal of Medical Research and Health Sciences, 2022; 5(5): 2013–2017. https://doi.org/10.52845/JMRHS/2022 -5-5-5.

