Congenital Hepatic Fibrosis Presented as an Isolated Liver Disease: a Case Report from Syria
Layal Ismail1,*, Muhsen Issa2, Taleb Mansour2, Amal Alhakim3 and Rana Issa4
1Department of Hepatology, Tishreen University, Syria
2Department of Hepatology, Al-Andalus university, Syria
3Department of pediatrics, Tishreen University Hospital, Syria
4Department of Pathology, Tishreen University Hospital, Syria
Received Date: 02/11/2022; Published Date: 18/11/2022
*Corresponding author: Layal Ismail, Department of Hepatology, Tishreen University, Syria
Abstract
Congenital Hepatic Fibrosis (CHF) is a rare disease that occurs in most cases in association with autosomal recessive polycystic kidney disease. CHF develops in the early embryonic stages as a result of ductal plate malformation and manifests clinically in any age, usually with signs of portal hypertension. In general, focal hepatic enlargement, normal liver function, and splenomegaly, along with renal involvement are telltale signs of CHF. In the absence of renal involvement, however, the diagnosis becomes more challenging and requires additional diagnostic procedures, such as liver biopsies. In this report, we discuss a case of a five-year- old child with a rare isolated form of CHF.
Keywords: Gastroenterology and hepatology; Pediatrics; Nephrology
Introduction
Congenital Hepatic Fibrosis (CHF) is a rare chronic liver disease caused by hepatic ductal plate malformation, with a prevalence of 1/10,000-20,000 [1]. The onset of clinical manifestations is variable, though it is most often diagnosed in adolescents and young adults. The symptoms are generally most severe when presenting in utero or during childhood and adolescence [2,3].
The current term “Congenital Hepatic Fibrosis“ was contrived by Kerr et al. in 1961 and was considered a fibropolycystic disease along with Caroli disease, Autosomal Dominant Polycystic Kidney Disease (ADPKD), and Autosomal Recessive Polycystic Kidney Disease (ARPKD) [2,4]. Some authors consider CHF a variant of ARPKD as it very rarely occurs without it, and that is supported by the limited number of isolated CHF cases in the literature [5,6].
CHF has no specific clinical presentation, and in most cases, symptoms of portal hypertension, such as varices and gastrointestinal bleeding, are the leading symptoms [2,5]. The wide spectrum of clinical presentations of isolated CHF in variable age groups makes its diagnosis difficult.
In this report, we discuss the case of a 5-year-old female child with isolated CHF. Our report aims to shed more light on the isolated form of CHF and to include it in the differential diagnosis of hepatic fibrosis in children.
Case Report
A 5-year-old female patient presented to the emergency department of Tishreen University Hospital with upper gastrointestinal bleeding. She was healthy until she developed a fever and a sore throat, so her parents gave her ibuprofen. Three days later, she started vomiting blood and discharging black stools. Physical examination revealed splenomegaly (4 cm below the left ribs), and a tender mass in the epigastric region. Laboratory tests showed a decrease in hemoglobin and platelet levels. Also, liver function tests were slightly elevated, whereas renal function tests were normal (Table 1).
Table 1: the results of the first laboratory test.

Blood was transfused to the child twice, and vitamin K and ranitidine were administered. When the patient became stable, Esophagogastroduodenoscopy (EGD) was performed, revealing esophageal varices; therefore, propranolol and omeprazole were added to the treatment. Color Doppler ultrasonography of the portal vein showed no abnormalities and no signs of portal vein thrombosis. Abdominal ultrasound confirmed the splenomegaly and revealed an enlargement of the left hepatic lobe. No renal abnormalities were discovered during imaging.
Laboratory tests were repeated after a while. Liver enzymes were back within normal range; however, thrombocytopenia persisted, and leukopenia was detected (Table 2), so the patient was enlisted for splenectomy. The later performed CT scan didn’t add any further information.
Table 2: The results of the second laboratory test.

To exclude neoplastic lesions, a liver biopsy was taken from the left lobe. Hematoxylin & eosin-stained sections showed disruption of the general architecture of the liver parenchyma by portal-portal fibrosis with occasional formation of nodules. The portal areas were expanded by fibrosis with minimal inflammatory cells and marked, irregular ductular proliferation. There was no cellular necrosis or atypia (Figure 1).
Our final diagnosis was CHF. Unfortunately, the patient left the hospital and returned to her hometown, so the treatment was interrupted.
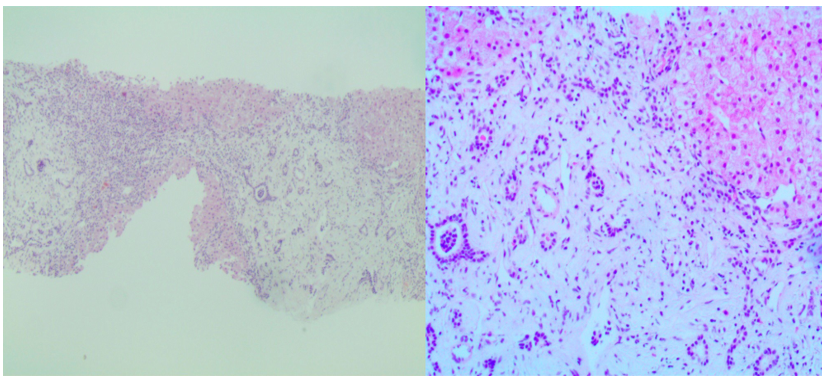
Figure 1: Microscopic findings of congenital hepatic fibrosis: expanded portal spaces by fibrosis with embedded proliferated ductules (H&E x40 and x 200).
Discussion
Congenital hepatic fibrosis is one of the chronic liver diseases in children that can cause portal and periportal fibrosis and, consequently, portal hypertension [7]. Intrahepatic diseases are the leading cause of portal hypertension in children, and CHF accounts for about 6.6% of them [8].
Recent studies have demonstrated that ciliopathies underlie several recessive and dominant malformation syndromes, including CHF [1,3]. Ciliopathies encompass a group of diseases that have similar but largely variable clinical phenotypes. They are caused by mutations that affect genes encoding structural or functional components of the primary cilia or basal bodies, such as PKHD1, which encodes fibrocystin, and TMEM67 [1,3].
Four types of CHF have been identified: portal hypertensive, cholangitic, mixed, and latent types [5]. In different studies, the portal hypertensive type with consequent bleeding varices is the most common. In our case, the little patient suffered from the complications of portal hypertension. Generally, the function of the liver parenchyma is not affected, therefore liver function tests remain intact, as in our case [4].
In most cases, CHF is associated with renal involvement, and this association makes the diagnosis easier, as the renal lesions can be easily detected through ultrasound [5]. In our case, though, neither ultrasound nor CT scan revealed any signs of renal abnormalities; furthermore, renal function tests were normal. Due to the lack of renal involvement, CHF eluded us, and our first differential diagnosis was hepatoblastoma, based on the presence of focal hepatic enlargement in the left lobe with an intact liver function. However, the results of the pathological examination of the biopsy, along with the clinical, radiological, and serological findings, excluded the neoplastic disease.
US and CT scans play a crucial role in diagnosing CHF and differentiating it from liver cirrhosis, but the golden test for confirming the diagnosis is the histological examination of the liver biopsy [1,2].
Ductal plate malformations can also lead to many other intrahepatic diseases such as Caroli’s disease, which affects large intrahepatic bile ducts [4]. The association between CHF and Caroli’s disease was reported in approximately 26% of the cases, while the isolated form accounted for 10% [9] In our patient, only the ductules were affected, so the association with Caroli’s disease was ruled out [5].
There is no specific drug that can cure CHF, and the treatment is directed toward treating complications and clinical symptoms. A liver transplant is recommended in the final stages of the disease [2].
Unfortunately, our little patient left the hospital, and the connection to her family was lost.
Our report aims to give more attention to the isolated form of congenital hepatic fibrosis and to include it in the differential diagnosis of chronic liver diseases in children, even in the absence of renal injury. The precise genetic basis of this rare entity is still unknown. Further studies are needed to elucidate its relation to polycystic kidney diseases and to determine if they are genetically separate.
Authorship Criteria
- Layal Isamil: Concept and design of study, interpretation of data, acquisition of resources, writing the study.
- Muhsen Issa: Designing and writing the study.
- Taleb Mansour: Concept and design of study.
- Amal Alhakim (guarantor author): Acquisitioning, analyzing and interpretation of the data.
- Rana Issa (guarantor author): Drafting the article and revising it critically for important intellectual content, analyzing of the data.
Conflicts of Interest Statement: There is no Conflict of interest.
Funding: There was no funding for this case.
References
- Zhu B, Du Z, Wang Z, Li Y, Zhang J, Zhu H. Congenital Hepatic Fibrosis in Children and Adults: Clinical Manifestations, Management, and Outcome—Case Series and Literature Review. Gastroenterology Research and Practice, 2020; 2020: 1-9. DOI: https://doi.org/10.1155/2020/8284274
- Shorbagi A, Bayraktar Y. Experience of a single center with congenital hepatic fibrosis: A review of the literature. World Journal of Gastroenterology, 2010; 16(6): 683-690. DOI: http://dx.doi.org/10.3748/wjg.v16.i6.683
- Vogel I, Ott P, Lildballe D, Hamilton-Dutoit S, Vilstrup H, Grønbæk H. Isolated congenital hepatic fibrosis associated with TMEM67 mutations: report of a new genotype-phenotype relationship. Clinical case reports, 2017; 5(7), 1098–1102. https://doi.org/10.1002/ccr3.981
- Desmet V. What is congenital hepatic fibrosis? Histopathology, 2007; 20(6): 465-478. DOI: http://dx.doi.org/1111/j.1365-2559.1992.tb01031.x
- Akhan O, Karaosmanoğlu A, Ergen B. Imaging findings in congenital hepatic fibrosis. European Journal of Radiology, 2007; 61(1): 18-24. DOI: http://dx.doi.org/10.1016/j.ejrad.2006.11.007
- Gunay–Aygun M, Font–Montgomery E, Lukose L, Tuchman Ge,rstein M, Piwnica–Worms K, Choyke P, et al. Characteristics of Congenital Hepatic Fibrosis in a Large Cohort of Patients With Autosomal Recessive Polycystic Kidney Disease. Gastroenterology, 2013; 144(1): 112-121.e2. DOI: http://dx.doi.org/10.1053/j.gastro.2012.09.056
- Pariente D, Franchi-Abella S. Paediatric chronic liver diseases: how to investigate and follow up? Role of imaging in the diagnosis of fibrosis. Pediatric Radiology, 2010; 40(6): 906-919. DOI: http://dx.doi.org/10.1007/s00247-010-1600-3
- Imanieh M, Dehghani S, Khoshkhui M, Malekpour A. Etiology of Portal Hypertension in Children: A Single Center’s Experiences. Middle East J Dig Dis, 2012; 4(4): 206–210. PMID: 24829658
- Cannella R, Giambelluca D, Diamarco M, Caruana G, Cutaia G, Midiri M, et al. Congenital Cystic Lesions of the Bile Ducts: Imaging-Based Diagnosis. Current Problems in Diagnostic Radiology, 2020; 49(4): 285-293. DOI: http://dx.doi.org/1067/j.cpradiol.2019.04.005

