Bowel Ischemia in a Child with Former Surgery and Acute Sars-Cov-2 Infection - A Rare Case in Current Pandemic
Hendrik Vossschulte1, Christine Foerster2, Stefan Eckert3, Lara Scheidler3, Jakob Popp3, Katrin Silkenbaeumer4, Dennis Nordhoff4, Eckard Hamelmann4, Winfried Barthlen1 and Sebastian Gaus5
1Department of Pediatric Surgery, Protestant Hospital of Bethel Foundation, University Hospital OWL, University of Bielefeld, Germany
2Institute of Pathology, Hospital Nord Stadt, affiliated with the University Hospital of the University of Bielefeld, Germany
3Department of Anesthesiology, Protestant Hospital of Bethel Foundation, University Hospital OWL, University of Bielefeld, Germany
4Department of Pediatrics, Protestant Hospital of Bethel Foundation, University Hospital OWL, University of Bielefeld, Germany
5Pediatric Emergency Department, Protestant Hospital of Bethel Foundation, University Hospital OWL, University of Bielefeld, Germany
Received Date: 01/10/2022; Published Date: 21/10/2022
*Corresponding author: Hendrik Vossschulte, MD, Department of Pediatric Surgery, Protestant Hospital of Bethel Foundation, University Hospital OWL, Campus Bielefeld Bethel, University of Bielefeld, Germany.
ORCID: 0000-0002-9699-9589
Abstract
Bowel ischemia in children is rare. Multiple factors may lead to such a disastrous event. An infection with SARS-CoV-2 may be a crucial factor in this reported case.
We present the case of a 6-year-old boy with an extensive ischemia of the small intestine. He has had a bladder resection due to rhabdomyosarcoma four years ago. Besides a former infection with SARS-CoV-2 one and a half years ago, he actually suffered from a new infection with the delta variant which was detected 10 days after admission.
About 65cm small intestine had been resected due to ischemia. The histopathological examination showed a long-segment hemorrhagic infarction of the jejunum.
Ischemia of the small intestine in a child must be considered especially with the history of former abdominal surgery. We see a correlation to an acute infection with SARS-CoV-2.
Keywords: SARS-CoV-2; Bowel ischemia; Children; COVID-19; Pandemic
Introduction
Bowel ischemia due to mesenteric thrombosis has been reported mainly in adult patients with a SARS-CoV-2 infection. In children, only few cases are reported [1]. Multisystem inflammatory syndrome in children (MIS-C) respectively pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) has been described since the beginning of the pandemic [2]. MIS-C could be located near to hyperinflammatory states like Kawasaki disease, toxic shock syndrome or macrophage-activation syndrome [3,4].
Case Report
We present a 6-year-old boy. He received a resection of the urine bladder and a channeled ileal conduit four years before because of a rhabdomyosarcoma of the bladder. In July 2020 he had symptoms of COVID-19 including loss of taste. There was no regular antigen or PCR testing at that time.
At time of presentation in January 2022 no vaccine was accepted for children of that age in Germany. The predominating virus types in Europe at that time were the wild type virus variant B and the variant BA.1.
At presentation in the evening hours, the boy had suffered from vomiting for four days. He was in a reduced dehydrated condition, had petechiae on the trunk, the abdomen was soft without signs for peritonitis. He had a tachycardia of 119 /min, a decrease of sodium of 124 mmol/l, c-reactive protein (CRP) value was 95.2 mg/l, leucocytes 4.5 /nl, lactate 3.1 mml/l, LDH 202 U/l, d-dimer levels increased from 1.95 to 3.71 mg/l within 7 hours, IL-6 9309 pg/ml. His temperature was 36.6 degrees, blood pressure 114/72 mmHg and oxygen saturation 97%. The conduit was vital, urinary excretion was reduced.
In the next morning, he was disoriented, respiratory and cardio circularly unstable (tachypnea > 50 /min, tachycardia 180 /min) and hypotensive (82/35 mmHg). Oxygen saturation was 98%. An emergency surgical intervention was indicated under the suspicion of a mechanical ileus.
During the anesthesiologic induction, the boy had a desaturation and circulatory arrest due to vomiting and bile aspiration. After 8 minutes of cardio-respiratory resuscitation and the necessary drugs he returned to spontaneous circulation.
During the following surgery, the mesentery of the small intestine showed scarring changes at the basis allegedly to the previous operation. Some vessels were thrombotic. More than 60cm the small intestine presented complete ischemic next to vital areas. There was no sign for a volvulus. In hope to preserve a longer distance bowel resection, an enterostomy about 60cm apart the Treitz Band was prepared with a planned second look operation. Due to the patient’s poor condition, the second look surgery was performed on the next day. Nothing of the bowel did recover, so about 65cm of the small intestine had to be resected (Figures 1-4).
At day 39, the enterostomy was relocated due to the now cachectic condition of the patient in hope for gaining weight besides the parenteral nutrition. The postoperative course was uneventful, and the boy left the hospital in a good condition.
Because of a suspected MIS-C, the boy was treated with immunoglobulins since day three after admission. Table 1 shows this medication. He was extubated five days after the operation. Afterwards he received non-invasive ventilation therapy because of a pneumonia while rising SARS-CoV-2 antibodies and a PCR testing clarified a now acute COVID 19 infection. Table 2 shows the timeline of the PCR testing and the testing for spike (S) and nucleocapsid (N) antibodies.
On day 17 after admission, he developed scaling of the fingertips (Figure 5) and repeated fever of unknown origin. On day 20, he showed a palmar exanthema (Figure 6).
One month after admission the respiratory support was stopped. Under the suspicion of MIS-C because of persisting subfebrile temperatures without rising infection values and without a clear focus, the boy received immunoglobulins (2g/kg) again and methylprednisolone 300mg/d for three days as recommended.
The cardiac function was measured as well functioning repeatedly.
The histopathological examination of the resected bowel showed a long-distancing and full-thickness hemorrhagic infarction of the bowel up to the edges of the resection (Figures 7, 8a, 8b). There was no vasculitis but multiple fibrin thrombi in the boundary zone of the blood vessels (Figures 9a, 9b). No virus was found in the ischemic bowel. There was no sign for recurrence of the malignancy.
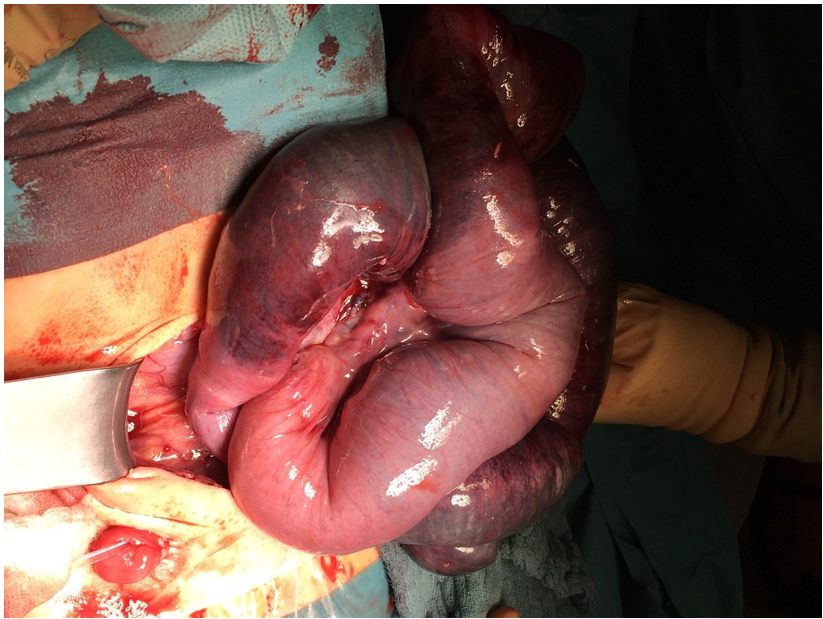
Figure 1: Ischemic aspect of the bowel during the first surgery. One can see the beginning on the right side of the picture and the ending on the left side. The ileal conduit is located in the right lower abdomen (lower left side of the picture).
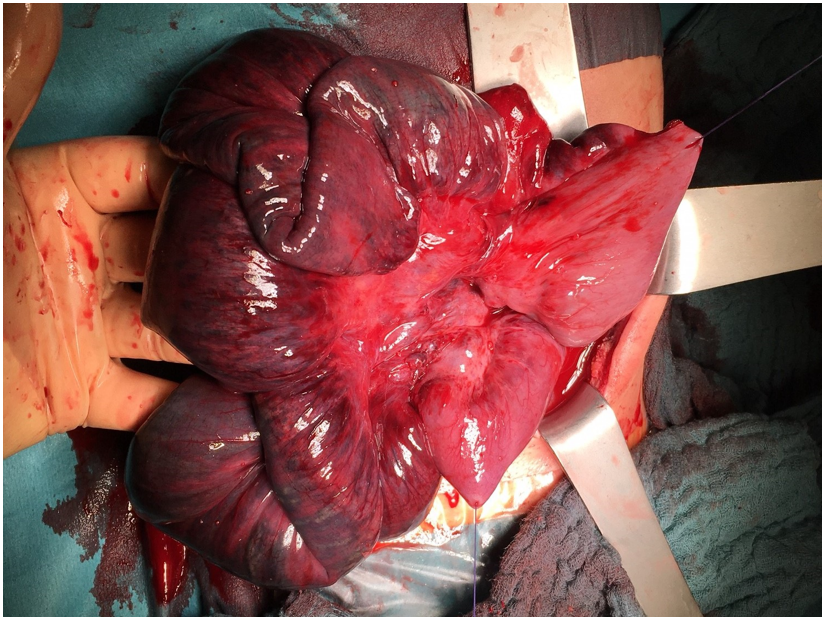
Figure 2: Another aspect of the ischemic bowel during the first surgery. One can see the dark blue with little pink vital aspect: the thickened and a little bit scarred mesentery. The two sutures are in the vital area before and after the ischemic one.
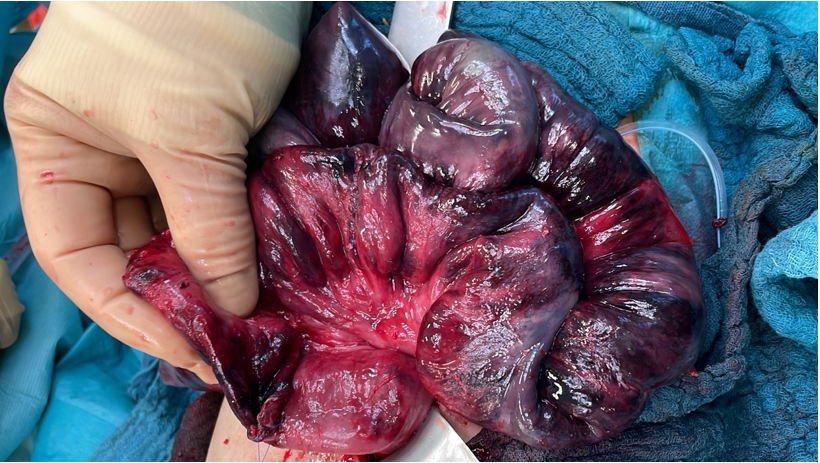
Figure 3: The ischemic aspect of the bowel on the next-day surgery. The suture at the bottom of the figure closes the former stoma.

Figure 4: Aspect of the lumen of the bowel at the external vital end of the resection. This resolved completely up to the time of the anastomosis of the bowel.
Table 1: Medication, first under the suspicion of MIS-C and then known acute SARS-CoV-2 infection.

Table 2: SARS-CoV-2 PCR testing compared to the antibodies found in the blood tests on different dates in the first month after hospital admission.
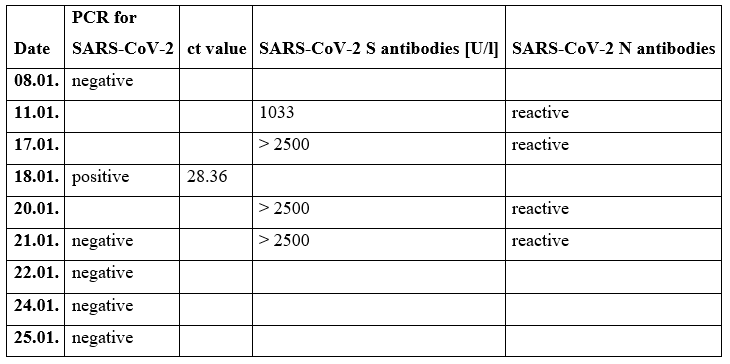
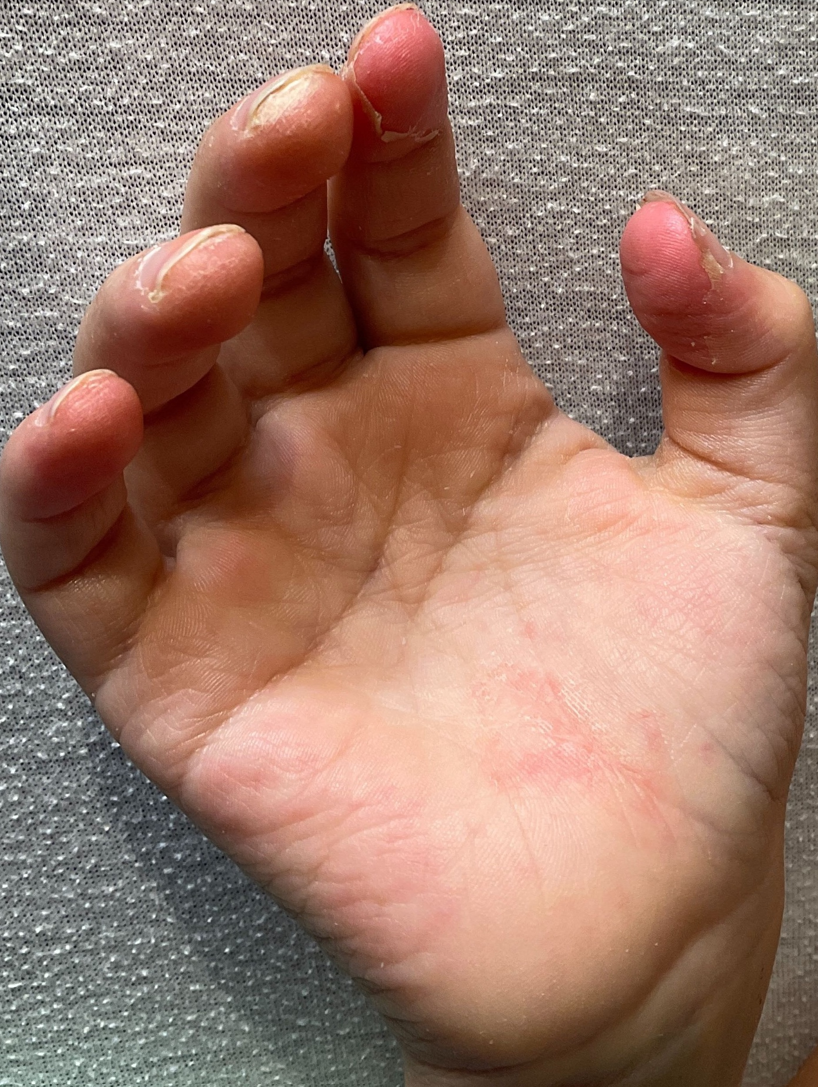
Figure 5: Scaling of the fingertips on day 17 after hospital admission.
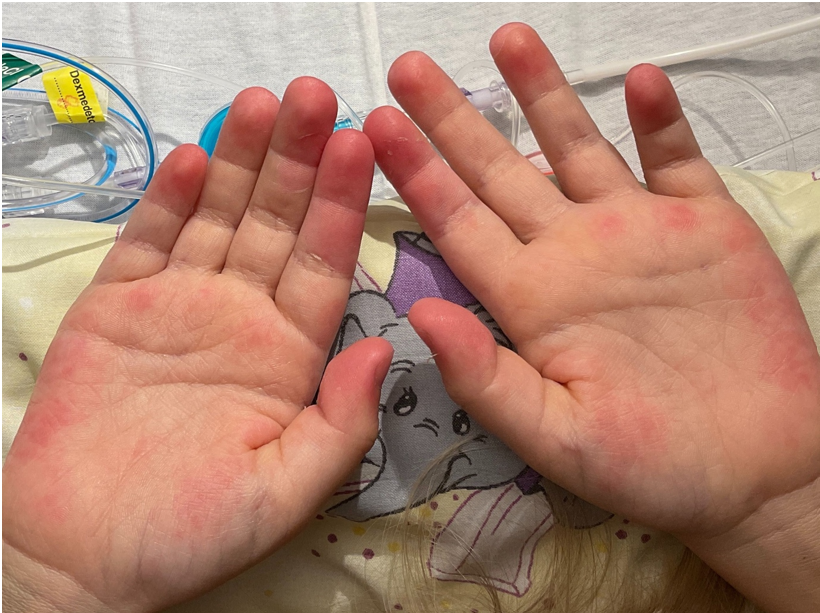
Figure 6: Palmar exanthema on day 20 after hospital admission.

Figure 7: Complete infarction of the small bowel with gray dusky and black discoloration; macroscopic view.
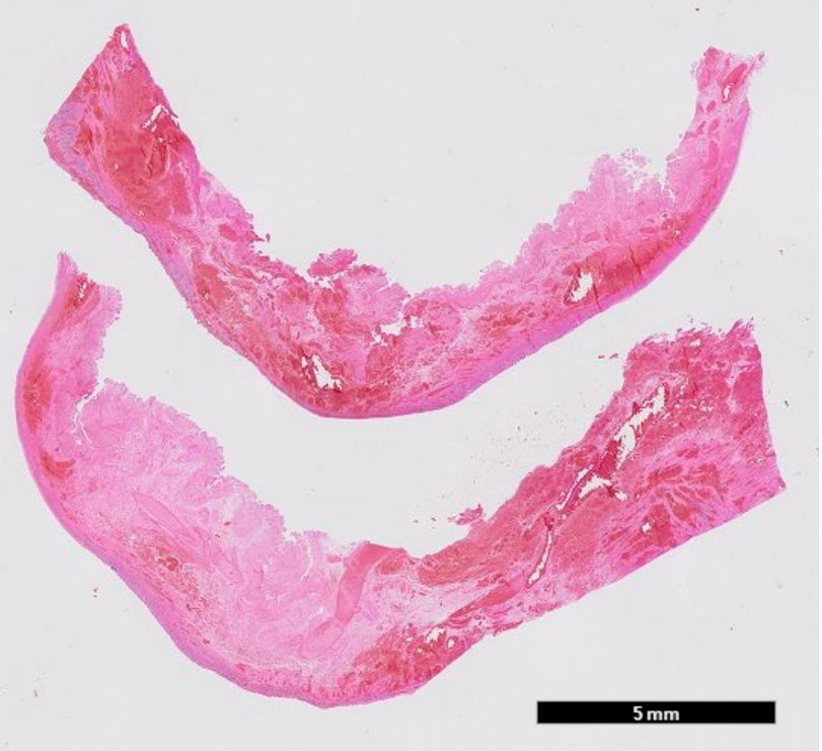
Figure 8a: Full thickness necrosis with hemorrhage in the mucosa, submucosa and muscularis propria; 5mm scale.
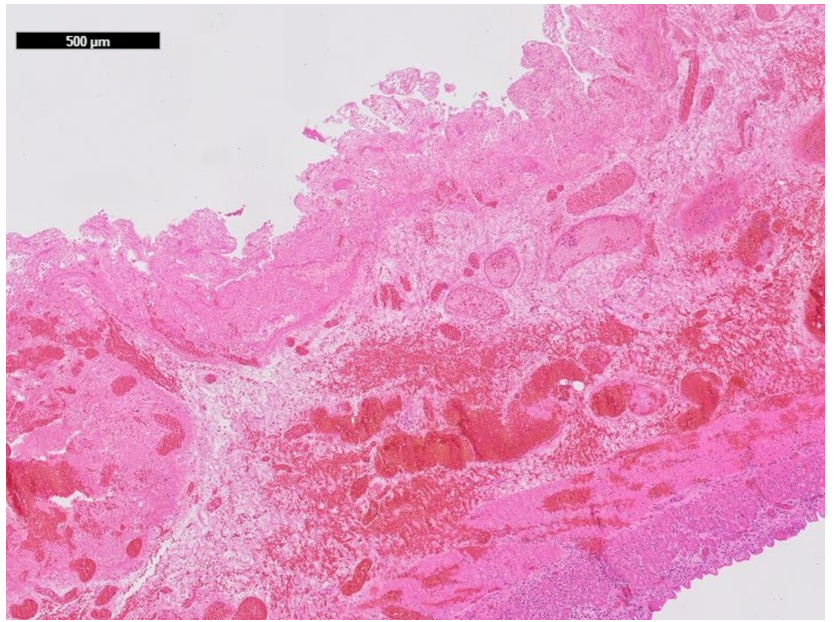
Figure 8b: Full thickness necrosis with hemorrhage in the mucosa, submucosa and muscularis propria; 500µm scale.
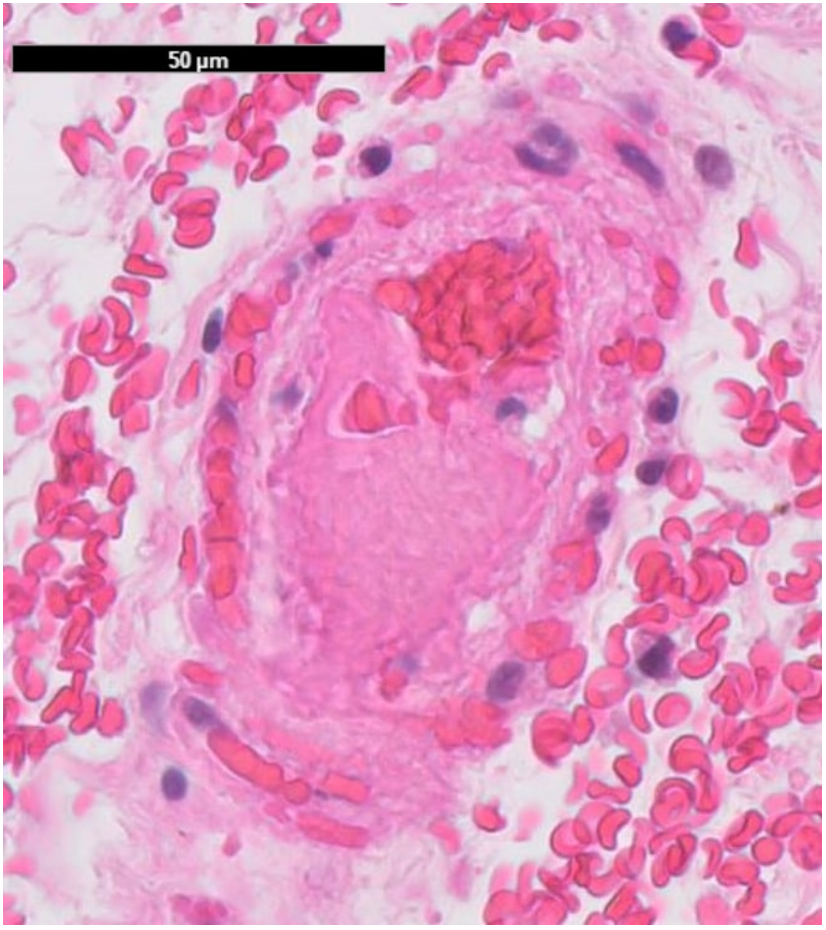
Figure 9a: Fibrin rich thrombus in a small sized vessel of the bowel mucosa. No significant inflammation; 50µm scale.
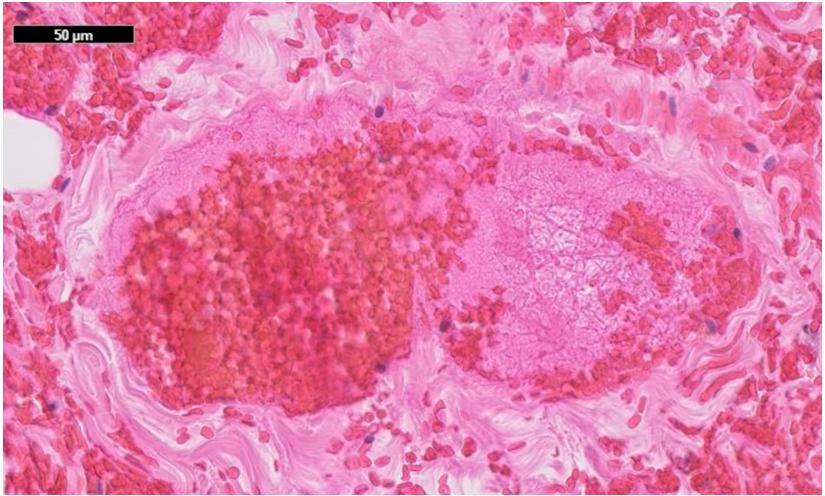
Figure 9b: Fibrin rich thrombus in a medium sized vessel of the bowel mucosa. No significant inflammation; 50µm scale.
Discussion
We present the rare situation of a bowel ischemia in a child. Additionally, to his previous abdominal surgery, he has a history of an assumed SARS-CoV-2 infection one and a half years ago and an additional actual SARS-CoV-2 infection. The predominating variant in Europe at that time was Omicron BA.1. The actual SARS-CoV-2 infection was undetected in the first days and had its peak about 10 days after admission. The boy developed a MIS-C three weeks later while slowly recovering from his acute infection and the surgery.
Acute mesenteric and bowel ischemia (AMI) about two weeks after a SARS-CoV-2 infection has been reported in an adult patient [5]. Our patient developed the bowel ischemia in the beginning of the SARS-CoV-2 infection.
The development of mesenteric ischemia or thrombosis and consecutive bowel ischemia in adult SARS-CoV-2 patients is a vicious circle [6-8]: The SARS-CoV-2 virus can directly damage bowel tissue by tropism of the virus in enterocytes via binding to angiotensin-converting enzyme-2 receptors on them. Additionally, hypercoagulability effects take place, leading to thrombosis of small vessels and bowel ischemia.
Hyper-inflammation in MIS-C differs from acute SARS-CoV-2 infection in adults whereas acute SARS-CoV-2 infection in children normally has a low-grade inflammatory response with mild or non-symptoms [9]. About 85% of the children with MIS-C develop gastrointestinal symptoms like abdominal pain, vomiting or diarrhea [3,9-11]. Mesenteric lymphadenopathy, enteritis, enterocolitis and bowel-wall thickening are reported as findings in CT scans in children with MIS-C. The median time from symptom onset to hospital admission was four days [12]. Although our patient’s first SARS-CoV-2 infection took place over one year ago, he presented with a mixture of symptoms like MIS-C and acute SARS-CoV-2 infection.
Several laboratory markers were identified to be possibly associated with an increased risk of a severe course of MIS-C, mostly C-reactive protein, D-dimer, ferritin, troponin, BNP and NT-pro-BNP [2,3,11]. In our patient, CRP and IL-6 levels initially were high in hindsight of the bowel necrosis. D-dimer levels, troponin and NT-pro-BNP were not measured in the initial phase but were high at the time point of the positive PCR testing ten days after admission. Cardiac function was appropriate all the time. MIS-C normally occurs one to six weeks after a SARS-CoV-2 infection [2,12,13], which fits with our patient’s fever and skin symptoms.
Khesrani et al. [1] reported the case of a 9-year-old girl with a history of medullar aplasia and the acute presentation of a pseudo appendicular syndrome that concealed an ileal ischemia. She developed a multisystem inflammatory syndrome associated with a SARS-CoV-2 infection. This girl had a pre-existing condition, too, but compared to our case no previous history of abdominal surgery. Malhotra et al. [14] speculated appendicitis as possible hyperinflammatory reaction within two weeks after an acute SARS-CoV-2 associated pneumonia in children.
Conclusion
The reason of developing bowel ischemia is unknown in this case. A context with the acute SARS-CoV-2 infection could be part of the cause even though no antibodies of SARS-CoV-2 in the bowel were found in the histopathological examination. It could also be assumed, that the first reactions to the virus took place in the bowel with previous operated and therefore damaged bowel mesentery just at the beginning of the infection while no virus could be detected in the PCR testing.
While recovering from surgery the boy had an acute SARS-CoV-2 infection with the high levels of ferritin, troponin, NT-pro-BNP like described for severe MIS-C [2]. If the assumed former SARS-CoV-2 infection played a role in this case remains unknown.
Another possibility could be an activation of the blood coagulation system and immune system because of the actual SARS-CoV-2 infection. While the former infection could have played a role in the speed of the actual reaction.
All authors contributed substantially to this manuscript.
The authors have nothing to disclose.
The authors received no specific funding for this work.
References
- Khesrani LS, Chana K, Sadar FZ, Dahdouh A, Ladjadj Y, Bouguermouh D. Intestinal ischemia secondary to Covid-19. J Pediatr Surg Case Rep, 2020; 61: 101604. doi:10.1016/j.epsc.2020.101604.
- Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health, 2021; 5(5): 323-331. doi: 10.1016/S2352-4642(21)00050-X. Epub 2021 Mar 10. PMID: 33711293; PMCID: PMC7943393.
- Esposito S, Principi N. Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Paediatr Drugs, 2021; 23(2): 119-129. doi: 10.1007/s40272-020-00435-x. Epub 2021 Jan 22. PMID: 33479801; PMCID: PMC7819738.
- Sood M, Sharma S, Sood I, Sharma K, Kaushik A. Emerging Evidence on Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection: A Systematic Review with Meta-analysis. SN Compr Clin Med, 2021: 1-10. doi: 10.1007/s42399-020-00690-6. Epub ahead of print. PMID: 33432304; PMCID: PMC7788276.
- Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior Mesenteric Artery Thrombosis and Acute Intestinal Ischemia as a Consequence of COVID-19 Infection. Am J Case Rep, 2020; 21: e925753. doi: 10.12659/AJCR.925753. PMID: 32724028; PMCID: PMC7417027.
- Chen C, Li YW, Shi PF, Qian SX. Acute Mesenteric Ischemia in Patients with COVID-19: Review of the literature. J Natl Med Assoc, 2022; 114(1): 47-55. doi: 10.1016/j.jnma.2021.12.003. Epub 2021 Dec 29. PMID: 34973847; PMCID: PMC8715336.
- Patel S, Parikh C, Verma D, Sundararajan R, Agrawal U, Bheemisetty N, et al. Bowel ischemia in COVID-19: A systematic review. Int J Clin Pract. 2021; 75(12): e14930. doi: 10.1111/ijcp.14930. Epub 2021 Oct 10. PMID: 34605117; PMCID: PMC8646310.
- Singh B, Kaur P. COVID-19 and acute mesenteric ischemia: A review of literature. Hematol Transfus Cell Ther, 2021; 43(1): 112-116. doi: 10.1016/j.htct.2020.10.959. Epub 2020 Nov 12. PMID: 33204997; PMCID: PMC7659807.
- Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr, 2021; 180(7): 2019-2034. doi: 10.1007/s00431-021-03993-5. Epub 2021 Feb 18. PMID: 33599835; PMCID: PMC7890544.
- Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health, 2020; 4(9): 669-677. doi: 10.1016/S2352-4642(20)30215-7. Epub 2020 Jul 9. Erratum in: Lancet Child Adolesc Health. 2020 Jul 17;: PMID: 32653054; PMCID: PMC7347350.
- Rafferty MS, Burrows H, Joseph JP, Leveille J, Nihtianova S, Amirian ES. Multisystem inflammatory syndrome in children (MIS-C) and the coronavirus pandemic: Current knowledge and implications for public health. J Infect Public Health, 2021; 14(4): 484-494. doi: 10.1016/j.jiph.2021.01.008. Epub 2021 Jan 18. PMID: 33743370; PMCID: PMC7813487.
- Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med, 2020; 383(4): 347-358. doi: 10.1056/NEJMoa2021756. Epub 2020 Jun 29. PMID: 32598830; PMCID: PMC7346766.
- Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med, 2020; 383(4): 334-346. doi: 10.1056/NEJMoa2021680. Epub 2020 Jun 29. PMID: 32598831; PMCID: PMC7346765.
- Malhotra A, Sturgill M, Whitley-Williams P, Lee YH, Esochaghi C, Rajasekhar H, et al. Pediatric COVID-19 and Appendicitis: A Gut Reaction to SARS-CoV-2? Pediatr Infect Dis J, 2021; 40(2): e49-e55. doi: 10.1097/INF.0000000000002998. PMID: 33298761; PMCID: PMC7855999.

