Severe Sinus Dysfunction Induced by Thalidomide
Sara Kadiri¹, Hanatou SEYDOU SADOU Maiga², Clarck Larsen Z Moumpala¹, Jamal Kheyi¹, Zouhair Lakhal¹ and Aatif Benyass¹
1Department of Cardiology of Military Hospital Mohammed V, Rabat / Morocco
2Department of Cardiology of University Hospital Center IBN SINA Rabat / Morocco
Received Date: 13/08/2022; Published Date: 12/09/2022
*Corresponding author: Sara Kadiri, Department of Cardiology of Military Hospital Mohammed V, Rabat / Morocco
Abstract
Background: One of the significant side effects of major doses of Thalidomide is Sinus dysfunction and it’s well described in the literature, it’s due to a disruption of the sinus node. this entity includes sinus bradycardia, sinoatrial blocks and sinus paralysis, sometimes requiring the implantation of a pacemaker.
Case presentation: We report the case of a 59-year-old man, who has in his medical history a mitral mechanical valve replacement, suffering from colonic angiodysplasia with melena for which he took 50 mg of thalidomide.
He presented with dizziness and fatigue in the emergency department of the Mohamed V military hospital in Rabat. An electrocardiogram performed found a 3rd degree sino-atrial block with a junctional escape rhythm at 35 cycles / minute while it was absent before the introduction of thalidomide.
Conclusion: Our observation elucidates one of a significant side effect of Thalidomide, particularly in patients with a maximum dose of 400 mg/day. Patients with a prescription of thalidomide should be closely monitored for signs and symptoms of bradycardia. This side effect was reduced by reducing the dose or stopping thalidomide, suggesting a reversible effect on the function of the sinus node.
Keywords: Sinus dysfunction; Severe bradycardia; Thalidomid; Cardiac conductive disorder; Sinus node; Sinus paralysis; Pacemaker; Temporary stimulation
Introduction
Sinus dysfunction is a major cardiac conductive disorder due to a disruption of the sinus node. this entity includes sinus bradycardia, sinoatrial blocks and sinus paralysis. its etiology is both intrinsic and extrinsic, sometimes requiring the implantation of a pacemaker.
We thus report the case of a 59-year-old patient, with a history of colonic angiodysplasia, presenting lower digestive hemorrhages, treated with thalidomide after a failure of electro-coagulation therapy, who presented an iatrogenic sinoatrial block.
Case Report
59-year-old patient, without cardiovascular risk factor, with history of mitral valve replacement with a mechanical prosthesis, anticoagulated with acenocoumarin, angiodysplasia of right colon with melena, treated by thalidomide 50 mg/day.
After failure of electro -coagulation therapy, the patient was admitted in the ICU for dyspnea stage III of New York Heart Association classification, dizziness and asthenia.
The clinical examination found a stable general condition, a pallor of the mucosa and the sclera. His hemodynamic constants are as follow: the blood pressure is 116/70 mm Hg, the heart rate was 35 beats/min, the respiratory rate was 27 cycles / min, the oxygen saturation was 100% in ambient air. He weighted 61 kg with a height of 175 cm (BMI 19.91 kg/m²).
The cardiac auscultation shows a regular rhythm, an audible prosthesis sounds, a rough, rasping systolic murmur with preserved B2 and a diastolic murmur along the left sternal border, there was no signs of congestive heart failure, there was not any externalized bleeding. The electrocardiogram showed a 3rd degree sino-atrial block with a heart rate of 35 cycles / minute. The trans-thoracic cardiac ultrasound found a normal mechanical mitral prosthesis, an ejection fraction of the left ventricle at 66%, an aortic moderate stenosis and regurgitation with massive functional tricuspid valve regurgitation.
The biological workup showed : Hgb 7.2 g / dl (VGM 75 fl, CCMH 30 g / dl), WBC 7000 / mm3, platelets 279,000 / mm3, PT 27%, INR 2.66, K+ 4.7 mmol / l; Na 137mmol/ l, urea 0.56 g / l, creatinine 10 mg / l, GFR 76 ml / min / m², HbA1c 5%, TSH 2.6 mIi / l, CRP 14 mg / l, troponin 60 ng / l, total cholesterol 1.08 g / l, Triglycerides 0.65 g/ l, LDL cholesterol 0.95 g / l, HDL cholesterol 0.40 g / l, normal liver function test.
For the hypochromic microcytic anemia, he was transfused with 4 units of red blood cells. His anticoagulant therapy was discontinued. The course was marked by the persistence of severe bradycardia although thalidomide was discontinued; the patient presented a heart rate up to 25 beats / minute, as well as lip thymic discomfort, thus requiring the introduction of atropine 1 mg intravenously then the implantation of a pacemaker.
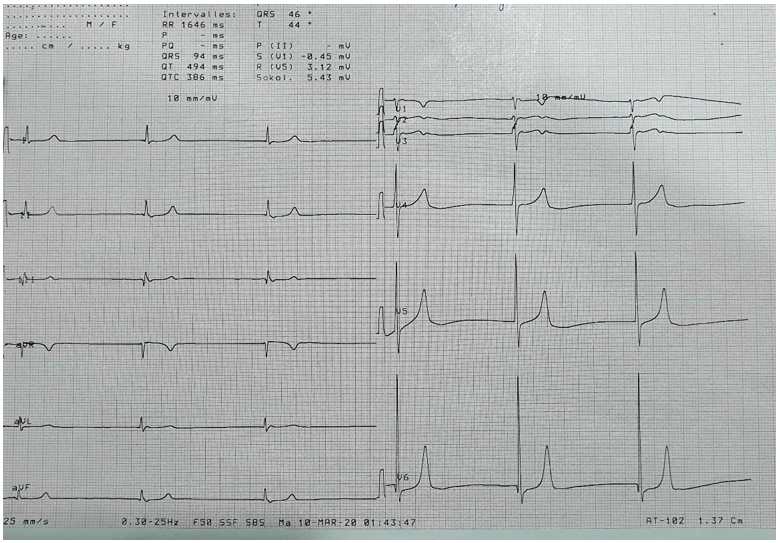
Figure 1: Electrocardiogram showing a 3rd degree sino-atrial block with a junctional escape rhythm at 35 cycles/minute.
Discussion
The mechanism of the occurrence of this conductive disorder is due to the fact that the nucleus of the vagus nerve is rapidly and completely inhibited by exposure to TNFa because thalidomide inhibits the expression and activity of TNF-a, thus inducing hyperactivity of the parasympathetic system and bradycardia [1].
Clinical trials have shown that thalidomide induces mild bradycardia (in 25 to 50% cases) and in some cases it can trigger severe bradycardia causing syncope or hypotension, only 1 to 3% of these patients needs a pacemaker [2].
Treatment with thalidomide should be started at a dose of 100-200 mg / day and increased weekly by 50-100 mg / day until the final dose of 400-800 mg / day is reached. The maximum tolerated dose should be administered. Patients receiving daily doses of 200 mg or less appear to tolerate treatment well with few side effects. However, almost all patients taking more than 400 mg / day have thalidomide-related toxicity. The latter is usually given at bedtime to minimize drowsiness and fatigue [1,3].
In our patient case, the maximum dose of thalidomide was 50 mg / day, the heart rate decreased sharply to 35 beats/min, resulting in dizziness, physical asthenia and dyspnea, the electrocardiogram performed, was in favor of sinus dysfunction. This latter could be mild, moderate or severe.
In severe cases, temporary stimulation is indicated [3]. In some patients, the sinus dysfunction would require a permanent pacemaker like in our patient whereas some patient could just be managed by discontinuing the thalidomide.
In a Spanish series carried out on patients suffering from hematological pathologies who were put on thalidomide 15.1% presented a sinus bradycardia under a daily dose going from 100 to 300 mg / day with a duration varying from 1 to 18 months. In all affected cases, the electrocardiogram showed sinus bradycardia with a heart rate between 32 and 48 beats per minute. The recovery time from normal heart rate ranged from 12 to 21 days after stopping thalidomide [4].
Our patient received thalidomide only for 20 days and at a dose of 50 mg / day, however, he presented with poorly tolerated persistent sinus dysfunction which ultimately required the implantation of a pacemaker. In the literature, at least a daily dose of 100 mg of thalidomide was required to cause bradycardia when our patient was only on 50 mg. Would this low threshold in our patient be accentuated by moderate aortic stenosis?
It should be noted that the prescription of thalidomide in patients suffering from cardiovascular disease whatever the etiology must be done with caution [5].
So, if patients are on thalidomide treatment, concomitant use of beta- blockers should be avoided and the EKG should be monitored regularly. Atropine helps improve the clinical signs associated with thalidomide-induced bradycardia.
Conclusion
Thalidomide-induced bradycardia appears to be a significant adverse effect, particularly in patients with a maximum dose of 400 mg/day. Thus, patients who have been placed on thalidomide should be closely monitored for signs and symptoms of bradycardia. This side effect was reduced by reducing the dose or stopping thalidomide, suggesting a reversible effect on the function of the sinus node. However, in severe cases with hypotension and syncope, temporary or even permanent stimulation should be considered as therapeutic option.
Conflict of Interest: None
References
- Shanbhag PS, Viswanath V, Torsekar Thalidomide: current status. Indian J Dermatol Venereol Leprol, 2006; 72: 75e80.
- Ghobrial IM, Rajkumar Management of thalidomide toxicity. J Support Oncol, 2003; 1: 194e205.
- Dimopoulos MA, Eleutherakis- Papaiakovou Adverse effects of thalidomide administration in patients with neoplastic diseases. Am J Med, 2004; 117: 508e515.
- Aguayo-González A, et Bradicardia asociada al uso de talidomida. Rev Invest Clin, 2006; 58(5): 424-431.
- Tseng CH, Su YJ, Lai Bradycardie induite par le thalidomide chez un vieil homme. Inter J. of Ger, 2010; 4(4): 197-198.

