A Rare Case of Giant Esophageal Leiomyoma Presenting with Massive Upper Gastrointestinal Hemorrhage
Harisankar AG, Rakesh Kumar Singh, Venkat Rao Chidipotu, Lajpat Agrawal and Tushar Saini
Department of Surgical Gastroenterology, Indira Gandhi Institute of Medical Sciences, India
Received Date: 04/08/2022; Published Date: 19/08/2022
*Corresponding author: Harisankar AG, Senior Resident (MCh Surgical Gastroenterology trainee), Department of Surgical Gastroenterology, Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India PIN 800014
Orcid ID: 000-0002-5632-8705
Abstract
Leiomyoma is the most commonly reported benign tumor of the esophagus. Most arise from the mid and lower esophagus. Usually, they are asymptomatic, however symptomatic leiomyoma usually presents with dysphagia, pain, and weight loss. Very rarely they might attain a size >10cm often referred to as Giant Esophageal Leiomyoma (GEL). Though most leiomyoma can be surgically managed by transthoracic enucleation, GEL often requires partial esophagectomy due to mucosal damage and malignant potential. Here, we report a case of a 26-year-old male with a giant esophageal leiomyoma of the distal esophagus extending up to the proximal stomach who presented with massive upper gastrointestinal hemorrhage and dysphagia which is very rarely reported in the literature. Complete excision of the tumor required distal esophagectomy with proximal gastrectomy and anastomotic repair was done with a gastric conduit
Keywords: Giant esophageal leiomyoma; Distal esophagectomy; Upper gastrointestinal hemorrhage; Transthoracic enucleation
Introduction
Although esophageal leiomyoma is rare, it accounts for the most common benign submucosal mesenchymal tumor of the esophagus. With an incidence of 8-43/10000 in autopsy series, it’s a rare neoplasm [1]. Most of the cases described are single lesions in the middle and distal esophagus and occur mainly in the third to fourth decade of life with a male preponderance [2]. Since most leiomyomas are smaller than 5cm they are asymptomatic and found incidentally. Occasionally sizes>10cm have been described in literature when they become symptomatic. Although transthoracic enucleation is a safe and effective approach in most cases of leiomyoma cases , the approach and management of GEL are different [3]. This is mainly because the mucosal and muscle defect cannot be repaired after simple enucleation of these giant tumors and in very rare instances they might have a sarcomatous change which can be missed [4]. This case report summarizes the rare clinical presentation of a GEL and its surgical experience
Case Presentation
A 26-year-old gentleman presented with dyspepsia and progressive dysphagia for 2 months. He was admitted to a local hospital with sudden onset of hematemesis and loss of consciousness. On evaluation, he was found to have a Blood pressure of 80/60mm Hg and a pulse rate of 112 per minute. His blood investigation revealed a hemoglobin value of 4.2gm/dl. One week later his dysphagia progressed and was completely intolerant to solid foods and was referred to our hospital for further evaluation. He had no episode of dyspnea, fever, or weight loss. He was not an alcoholic or smoker and his family history was insignificant. General examination was unremarkable except that he had mild pallor. Fresh blood investigations revealed hemoglobin of 9.5gm/dl and a total leukocyte count of 8300cu/mm. Contrast-Enhanced Computer Tomography (CECT) of the thorax revealed a large lobular (11x10x7 cm) non-enhancing homogenous exophytic lesion compressing and displacing the lower esophagus and gastroesophageal junction extending to the cardia of the stomach and causing marked luminal narrowing. The lesion anteriorly abuts the pericardium, laterally the left lung parenchyma, and superiorly the left pulmonary vein (Figure 1, panels a,b)
Upper gastrointestinal endoscopy showed an esophageal submucosal mass with a luminal compromise with dilated, tortuous vessels over it. Based on these findings a differential diagnosis of Gastrointestinal Stromal Tumor (GIST) or leiomyoma was made. The patient was taken up for surgery after a proper anesthesia workup. He was positioned in a right lateral decubitus position and a left thoracotomy incision was made through the seventh intercostal space. The same incision was extended to an upper midline laparotomy incision. We found a hard tumor in the mid esophagus in the posterior mediastinum extending from 2 cm below the carina and extending below to the cardia of the stomach involving the proximal stomach (Figure 2, panel a) The tumor was found adherent to the inferior pulmonary ligament, left vagus nerve and hemiazygous vein. We proceeded with distal esophagectomy combined with proximal gastrectomy. Proximally esophagus was divided 2cm below left pulmonary vein and distally stomach was divided 4 cm below the gastroesophageal junction. Gastric conduit was then created along the greater curvature and esophagogastric anastomosis was performed hand sewn in two layers (Figure 2, panel b)
Feeding jejunostomy was also done by Witzel technique. A 28 Fr soft thoracic catheter was placed in the thoracic cavity close to the anastomosis. Postoperative day two jejunostomy feeding was initiated. Postoperative day four, gastrograffin swallow study was performed in which normal anastomotic patency was ascertained (Figure 3) Hence, we started on oral fluids on the same day and was discharged on postoperative day eight after drain removal. He remains symptom free at five months of follow-up and tolerating both solid and liquid diet.
Gross pathological examination revealed a well encapsulated grey brown globular mass of size 12x8x7cm with areas of hemorrhage at centre (Figure 4, panel a,b). The histopathological examination showed unremarkable spindle cells arranged in whirls and fascicles with no atypical mitosis or necrosis (Figure 5). Immunohistochemistry (IHC) revealed positive staining for Smooth muscle actin (SMA), Desmin, Caldesmon and Ki67 index of 3 % (Figure 6,panel a,b,c,d).On the basis of histopathological finding and IHC, a diagnosis of leiomyoma was made.
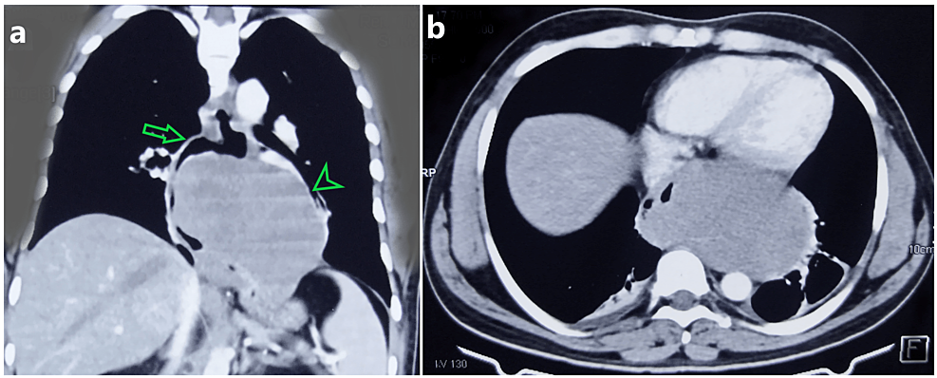
Figure 1: Contrast-enhanced computer tomography images.
(a) Coronal section showing homogenous non enhancing lobulated lesion involving distal esophagus (arrow) which closely abuts the left pulmonary vein and lung parenchyma (arrow head)
(b) Axial section showing the tumor closely abutting the pericardium anteriorly and displacing the esophagus laterally

Figure 2: Intraoperative images.
(a) Intraoperative image showing tumor extending from the distal part of the middle esophagus (arrow) and below across the gastroesophageal junction to the cardia of the stomach(arrowhead)
(b) Intraoperative image showing gastric conduit prepared and the esophagogastric anastomosis (arrow) performed
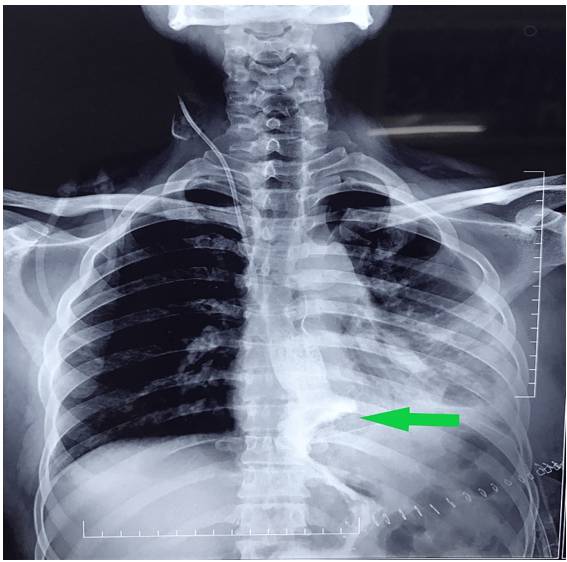
Figure 3: Postoperative gastrograffin swallow study.
Gastrograffin swallow study performed on postoperative day four showing the patent esophagogastric anastomotic site (arrow)

Figure 4: Resected tumor specimen: gross pathological features.
(a) A resected specimen showing well - encapsulated neoplasm with areas of hemorrhage
(b) Tumor with attached part of the proximal stomach (arrowhead)
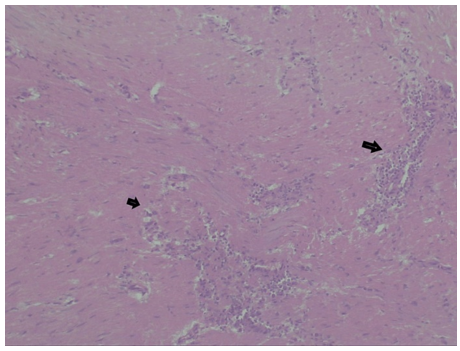
Figure 5: Histopathological image (magnification 40X).
Hematoxylin and eosin staining of the tumor cells reveal unremarkeable spindle cells arranged in fascicles with no atypical mitosis

Figure 6: Immunohistochemistry images (magnification 40X).
Tumor cells (a) Positive for (SMA); (b) positive for Desmin ; (c) positive for Caldesmon; (d) expressing Ki67 index of 3%
Discussion
Leiomyoma is the most common benign tumor of the esophagus. Most commonly it originates from the muscular layer of the mid and lower esophagus. Benign tumors account for only 10% of all esophageal tumors and leiomyoma accounts for hardly 4% among them [5]. Leiomyoma usually is asymptomatic or may present with non-specific symptoms like dysphagia, pain, and pyrosis. Less commonly they may present with symptoms that include weight loss, hematemesis, or melena. Bleeding is a much more common symptom in gastric leiomyoma. In the present case patient presented with massive hematemesis and dysphagia which is rather uncommon. It can occur at any age, but 90% of them occur in the third to fourth decade of life with a proportional male to female incidence of 2:1 [6].
Radiological evaluation with a barium swallow, CECT, Upper gastrointestinal endoscopy, and endoscopic ultrasound may be useful to clinch the diagnosis [7]. CECT best describes the location, extent, and relation with surrounding structures. In the present case esophagoscopy showed an esophageal submucosal mass with a luminal compromise with dilated, tortuous vessels over it. However, the characteristic finding that has been described is esophageal narrowing with intact overlying mucosa [8]. A preoperative biopsy is however not mandatory since there is a risk of mucosal bleed. In most of the cases, biopsy material was insufficient to establish an accurate histopathological diagnosis [3].
Most leiomyomas are intramural and well encapsulated hence can be treated by enucleation. Up to 8 cm leiomyoma has been treated by enucleation provided there is a tension-free approximation of myotomy and that mucosa is intact. Proper approximation of the muscle layer of the esophagus is needed to maintain the propulsive activity of the esophagus and this prevents reflux esophagitis in long term [3,7]. Approximately 5 % of them are giant leiomyoma defined as size >10cm [9]. Large leiomyoma is often difficult to excise without mucosal breach due to tight and extensive adhesion. These factors further necessitate the need for esophagectomy over enucleation. Transformation of leiomyoma to sarcoma is also a rare possibility reported in <0.2% of cases. Hence esophagectomy should be done to distinguish benign from malignant neoplasm in GEL [10]. Esophagectomy is mostly done with gastric pull-up through a left posterolateral thoracotomy approach [7]. In our case, we performed the procedure through a single left thoracoabdominal incision. The main limiting factor for a thoracoscopic approach is the creation of a plane between the tumor and mucosa, albeit minimally invasive enucleation has also been reported for GEL [11].
Several reconstruction techniques have been described for esophageal reconstruction. Among them, gastric and colonic conduits have gained importance. The choice of conduit depends on the location of the tumor. The gastric pull-up is widely accepted because of its robust blood supply and requirement of only a single anastomosis. Colonic conduit is preferred by some authors when the tumor is located in the proximal part of the esophagus with the rationale of reducing the acid reflux when the whole stomach is used for anastomosis [12,13]. However gastroesophageal reflux has been significantly reduced by gastric tubulization along the greater curvature and maintaining at least 6cm of the gastric tube below the diaphragm [4]. Our case was exceptional since the tumor was extending to the cardia of the stomach and hence had to proceed with distal esophagectomy and proximal gastrectomy. This procedure has been described for benign tumors of the gastroesophageal junction by using isoperistaltic jejunal interposition by Merendino et al [14]. Although this procedure delivers a reliable antireflux mechanism, it’s a complex procedure requiring three anastomoses. Hence reconstruction of cardia with a gastric conduit was tried in our case. However, a comparison between the two procedures has shown that the Merendino procedure was associated with lower anastomotic leak and shorter hospital stay than the gastric conduit procedure [15]. However, we used a gastric conduit for anastomosis and the patient was discharged on postoperative day eight after tolerating a proper oral diet.
Conclusions
GEL is a rare entity that presents many diagnostic and surgical challenges. Studies describing the preferred surgical approach are very scarce. This case report provides an insight into an uncommon presentation of massive upper gastrointestinal bleed which often causes a diagnostic dilemma. Due to its large size, enucleation technique is rarely feasible. It is also difficult to identify the underlying malignant nature of GEL preoperatively, which ultimately depends on the histopathological and immunohistochemical analysis of complete resected specimen. In the present case complete resection of the tumor required distal esophagectomy with proximal gastrectomy which was safely repaired with a gastric conduit.
References
- Yang PS, Lee KS, Lee SJ, et.al: Esophageal leiomyoma: radiologic findings in 12 patients. Korean J Radiol, 2001; 2: 132-137. 3348/kjr.2001.2.3.132.
- George FH, Laura WH, Kathryn FH, Gregory BD, Blanchard DK, Roger SF, et al. Tumors of the esophagus. World J Surg. 2000, 4:401-11. 1007/s002689910065.
- Bonavina L, Segalin A, Rosati R, Pavanello M, Peracchia A: Surgical therapy of esophageal leiomyoma. J Am Coll Surg. 1995; 181(3): 257: 62.
- De Giacomo T, Bruschini P, Arcieri S, et.al: Partial esophagectomy for giant leiomyoma of the esophagus: report of 7 cases. Eur J Cardiothoracic Surg, 2015, 47:143-145. 1093/ejcts/ezu146.
- Seremetis MG, Lyons WS, deGuzman VC, Peabody JW Jr. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer. 1976, 5:2820380547-3. 1002/1097-0142(197611)38:5<2166: aid-cncr2820380547>3.0.co;2-b.
- Lee LS, Singhal S, Brinster CJ, et.al: Current management of esophageal leiomyoma. J Am Coll Surg, 2004; 198: 136-146. 1016/j.jamcollsurg.2003.08.015.
- Mutairi H, Al-Akkad M, Afzal M, Chaudhry I. Giant leiomyoma of the esophagus with eosinophilic infiltration. BMJ Case Rep. 2013, 2013: 2013201343. 1136/bcr-2013-201343.
- Aurea P, Grazia M, Petrella F, Bazzocchi R. Giant leiomyoma of the esophagus. Eur J Cardiothoracic Surg, 2002; 22: 1008-1010. 1016/s1010-7940(02)00569-9.
- Cheng BC, Chang S, Mao ZF, Li MJ, Huang J, Wang ZW, et al. Surgical treatment of giant esophageal leiomyoma. WorldJGastroenterol. 2005; 11: 4258-4260. 3748/wjg. v11.i27.4258.
- Coskun A, Unubol M, Yukselen O, et.al. Esophageal Leiomyoma in Patients with Megaloblastic Anemia. Euroasian J Hepatogastroenterol. 2014; 4: 98-100. 5005/jp-journals-10018-1110
- Chen X, Xi Y, Wang H, Tan L. Minimally invasive surgery for giant esophageal leiomyoma: a case report & review of the literatures. J Thorac Dis. 2017; 9: 26. 21037/jtd.2017.01.34
- Cheng BC, Chang S, Mao ZF,et.al: Surgical treatment of giant esophageal, 2005; 4258-4560. 3748/wjg. v11.i27.4258.
- Pearson FG, RD. Henderson Experimental and clinical studies of gastroplasty in the management of acquired short esophagus: Surg. Gynecol. Obstet. 1973; 136: 737-744. 1016/0022-3468(74)90048-7.
- Merendino KA, Dillard DH: The concept of sphincter substitution by an interposed jejunal segment for anatomic and physiologic abnormalities at the esophagogastric junction; with special reference to reflux esophagitis, cardiospasm and esophageal varices. Ann Surg, 1955; 142: 486-506. 1097/00000658-195509000-00015
- Eichelmann A-K, Nikitina M, Bahde R, et.al: Merendino Resection vs. Transhiatal Gastric Conduit After Resection of the Cardia and the Gastroesophageal Junction. The American Surgeon, 2022; 88: 194-200. 1177/0003134820983185.

