Porphyria Cutanea Tarda, Type 2, in a Man with a Novel UROD Mutation
Stephen Soufleris1, Michelle Moore PA-C1, John D Phillips2, Brian Netzel3, Sean Rudnick1, Denise Faust SOCRA1 and Herbert L Bonkovsky1
1Section on Gastroenterology and Hepatology, Department of Medicine, Wake Forest University/NC Baptist Medical Center, USA
2Division of Hematology, Department of Medicine, University of Utah Health Science Center, USA
Received Date: 30/07/2022; Published Date: 12/08/2022
*Corresponding author: Stephen Soufleris, MD, 1Section on Gastroenterology and Hepatology, Department of Medicine, Wake Forest University/NC Baptist Medical Center, Winston-Salem, NC, 27157, USA
Abstract
We report on a man in his seventh decade with Porphyria Cutanea Tarda (PCT) caused by a novel mutation in Uroporphyrinogen Decarboxylase (UROD). Genetic testing revealed a novel mutation of UROD gene (c.224 G>C; p. Arg 75 Pro) with red blood cell UROD activity decreased by 50%. We demonstrate that mutation in UROD is predicted to have a major effect on protein structure and function.
Keywords: Cutaneous porphyria; Heme; Iron; Uroporphyrinogen decarboxylase; Uroporphyrin
Introduction
Porphyria Cutanea Tarda (PCT) is usually caused by acquired critical defects in Uroporphyrinogen Decarboxylase (UROD) activity in the liver [1]. About 20% of cases occur in patients who have a partial decrease in UROD activity that is genetically determined. We describe a case of a man with a novel UROD mutation causing PCT type 2.
Case Report
A 77-year-old man was evaluated for 6 months of painful, bullous blistering over the dorsal surfaces of both hands and forearms and, to a lesser extent, his head (Figure 1A). The rash initially appeared during intermittent episodes but became more frequent and persistent over time. His medical history was notable for hypertension, melanoma, and stroke. He reported a long history of heavy alcohol and tobacco use. He reported no known family history of porphyria
He worked for many years as the owner of a manufacturing facility, with exposure to various chemicals including cooling agents, lubricants, weed killers, various oils and chemicals used to shine metals.
Medications at the time of his initial visit included the following: aspirin, cholecalciferol, vitamin B-12, omega-3 fatty acids, fish oil, simvastatin, and tamsulosin.
Physical examination revealed tense bullae with milia and hyperpigmented macules on dorsal hands and forearms bilaterally. Biopsies of his right hand revealed a subepidermal blister containing a small number of neutrophils with fibrin. There was also evidence of festooning of the vessels into the blister cavity. Direct immunofluorescence was negative for IgA, IgG, IgM, and complement (C3). This biopsy was consistent with porphyria.
Labs revealed serum aspartate aminotransferase 63 U/L, alanine aminotransferase 64 U/L, alkaline phosphatase 54 U/L, and total bilirubin 0.8 mg/dL. Iron profile was normal, and antibodies against HIV and hepatitis A, B and C were negative. HFE genetic testing revealed C282Y -/- ; H63D +/-. Total plasma porphyrins were elevated to 13.4 mcg/dL. Fractionated urine, stool, and plasma porphyrins were obtained [Table 1]. Urinary uro- and hepta-carboxyl porphyrins were markedly elevated, consistent with PCT [2]. The patient was advised to start hydroxychloroquine 100 mg 3 times weekly and avoid alcohol, tobacco use, and sun exposure. Due to severity and slow improvement, iron reduction therapy was added at month 7.
Genetic testing revealed a novel mutation of UROD gene (c.224 G>C; p. Arg 75 Pro) with red blood cell UROD activity decreased by 50%.
The patient returned to the clinic at 3 and 7 months after the index visit. He indicated that he had stopped all alcohol use but continued to smoke cigarettes. The rash on his hands had improved (Figure 1B). Repeat urine and serum porphyrins showed improvement (Table 1). The mutation in UROD is predicted to have a major effect on protein structure and function (Figure 2).
Table 1: Results of Selected Laboratory Studies.
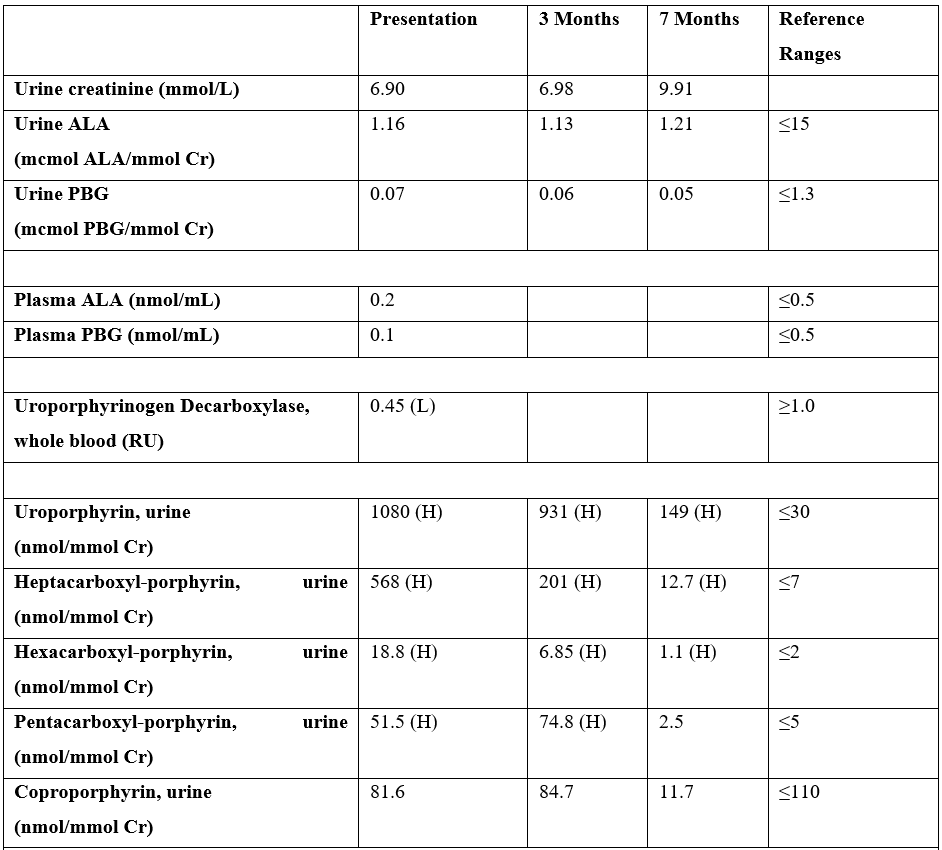
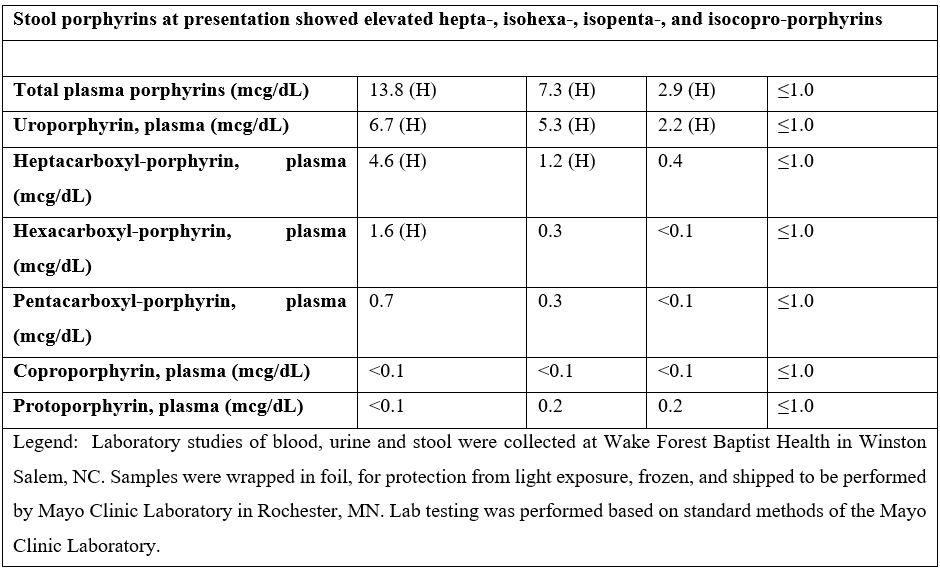
Figure 1: A. Dorsal right hand at presentation. B. Dorsal right hand at 7-month follow-up.

Figure 2: Structure of URO-D showing the substrate (yellow) in the active site at the C-terminal end of the beta-sheets (magenta) that form the core of the TIM-barrel structure. Arginine 75 (Arg 75), shown in green, is the penultimate residue in one of the eight corresponding alpha-helices surrounding the central core. Arg 75 forms two hydrogen bonds with glutamic acid 45 (Glu 45) of the preceding helix in the structure. This hydrogen-bonding network supports the tertiary fold of the protein in this otherwise solvent-exposed region. The patient’s UROD mutation (Arg 75 Pro) disrupts the helix with premature termination and eliminates the hydrogen-bonding network.
Discussion
Inherited partial deficiency in UROD is not sufficient to cause clinically manifest PCT. Other factors that increase oxidative stress are also required [3,4]. Chief among these is alcohol, iron, smoking, hepatitis C virus and estrogens [3]. This patient harbors a mutation in UROD gene not heretofore described in PCT type 2, but that we predict and show markedly affects the structure and activity of the protein product [Figure 2] [5]. Nonetheless, even with this mutation, the patient did not develop clinically manifest PCT until age 77, emphasizing the importance of other environmental and acquired factors in disease pathogenesis.
Authorship Criteria
Stephen Soufleris MD – Acquisition of data, drafting article, revising article. Guarantor Author
Michelle Moore PA-C – Acquisition of data, revising article
John D. Phillips PhD – Acquisition of data, intellectual content, revising article
Brian Netzel MS – Acquisition of data, revising article
Sean Rudnick MD – Analysis and interpretation of data, revising article
Denise Faust SOCRA – Acquisition of data, revising article
Herbert L. Bonkovsky MD – Analysis and interpretation of data, revising article, intellectual content.
Informed Consent: A signed statement of informed consent was obtained for publication of the details of this case.
Conflicts of Interest: None to disclose
Grant Information: This work was supported by a cooperative agreement with NIDDK/NIH 2 U 54 DK 083909, NIDDK/NIH DK 020503, Wake Forest University CTSI UL1TR001420, and by Protect the Future Funds from the American Porphyria Foundation.
References
- Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med, 2017; 377(9): 862-872.
- Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab, 2019; 128(3): 271-281.
- Caballes FR, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int, 2012; 32(6): 880-893.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, Obando J, Di Bisceglie A, Tattrie C, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology, 1998; 27(6): 1661-1669.
- Badminton MN, Whatley SD, Sardh E, Aarsand AK. Porphyrins and the porphyrias. In: Rifai N, Horvath AR, Wittwer C, eds. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics. 6th ed. St. Louis: Elsevier, 2018; 776-799.

