Case Report on Traumatic Brain Injury – Management in the African Context
Reginald Ononye1,*, Terngu Moti2 and Ifeyinwa Ogbogu3
1Department of Neurosurgery, Brighton and Sussex University Hospitals, Brighton, England
2Department of Neurosurgery, Salford Royal NHS Foundation Trust, Manchester, England
3Final year Medical Student, College of Medicine, University of Ibadan, Nigeria
Received Date: 25/05/2022; Published Date: 10/06/2022
*Corresponding author: Reginald Ononye, Clinical Fellow, Neurosurgery, Brighton and Sussex University Hospitals, Brighton, England
Abstract
Traumatic brain injury is a major medical and socio-economic issue worldwide, more importantly in resource-poor countries where adequate and invasive monitoring is a major challenge as well as infection control. It is said to be the leading cause of mortality among children and young adults worldwide [1,2,3,4].
The severity of traumatic brain injury is graded into mild, moderate and severe based on Glasgow coma scale score and it is a good predictor of outcome [5,6].
Traumatic brain injury carries significant morbidity and mortality. However, recovery over the last 2 decades has improved owing to aggressive efforts to mitigate secondary brain injury, maintaining neurological homeostasis and averting raised intracranial pressure [3,4].
A case of a middle-aged man who sustained multiple trauma and head injury following an assault by armed robbers is reported from National Hospital, Abuja, Nigeria. Presenting GCS score and post-resuscitation was 15/15 (E – 4, V – 5, M – 6) however, that progressively deteriorated to 6/15 (E – 2, V – 2, M – 2) warranting intensive care unit admission, endotracheal intubation, mechanical ventilation and inotropic support. The patient’s clinical status remained poor until his demise (brain death) 3 days post trauma in the ICU.
The management of traumatic brain injury is multidisciplinary for optimum outcome. Increasing evidence pioneered by the Brain Trauma Foundation has shown that Clinical Practice Guidelines (CPG) with clear key management goals may improve recovery and rapid return to significant or full functional premorbid state. Randomized controlled clinical trials (double-blind) to test and validate current management practices for traumatic brain injury, and collaborative scientific research are necessary to advance and improve trends in managing traumatic brain injury.
LMIC (resource-poor) face peculiar challenges in the management of TBI. The development of Clinical Practice Guidelines (factoring in technical difficulties and resource limitations) based on highest level of scientific evidence available may improve outcome/survival of head injured patients. This paper is a call-to-action to the need to standardize management and develop CPG specific for developing/resource-poor setting.
Introduction
Traumatic Brain Injury (TBI) is not an uncommon presentation in the emergency room. It is a major medical and socio-economic issue worldwide especially in developing countries. TBI is the leading cause of mortality and morbidity among children and young adults [1,2,3,4]. Common aetiologies leading to this condition include: Road traffic accidents, falls, assault and sports related injuries [1].
The incidence of TBI in developing countries, like Nigeria is largely unknown. However, UK based studies have shown that about 1million patients present to the hospital having suffered head injury with almost one-half, 16years and less, and a vast majority having mild to moderate head injuries [1,4]. Males have been found to be 2 to 3 times more likely to suffer TBI when compared to females [1,4].
Traumatic brain injury can be classified into mild >13, moderate 9 – 13, and severe 3 – 8, based on the Glasgow coma scale (GCS) score – most common method of assessing severity of TBI – post-resuscitation. The GCS score is predictive of outcome [1,2,4,7,8].
Glasgow Coma Scale (Hutchinson Clinical Methods 23rd edition)
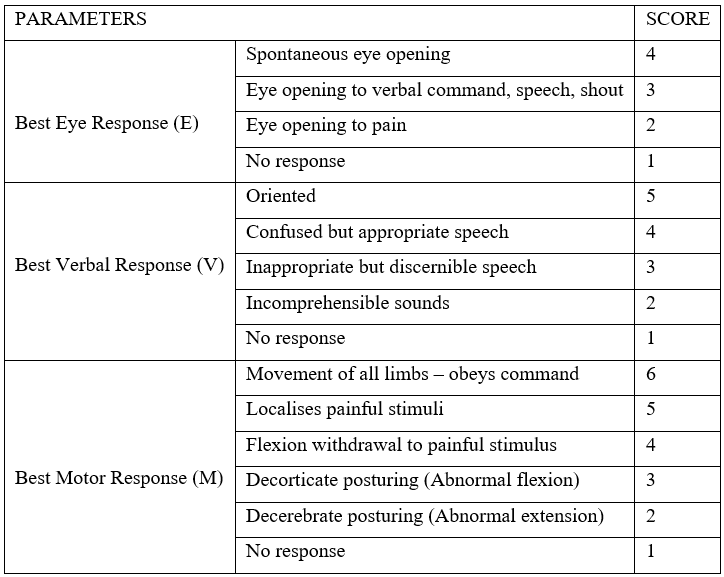
Traumatic brain injury occurs in 2 distinct phases: Primary brain injury (sustained at the time of injury, more or less irreversible) and secondary brain injury resulting in further neuronal damage [4,8].
The management of TBI is multidisciplinary for best outcome and involves a team of neuro-intensivist (anaesthetist in Nigeria), neurosurgeon, neurologist, specialized nurses, respiratory and physical therapist, nutritionist and other support/technical staff. Critical care management is paramount in order to maintain in homeostasis as much as possible, the physiologic processes of the body.
Mortality from TBI has consistently improved over time. This improvement is due to aggressive efforts to mitigate secondary brain injury, maintenance of neurological homeostasis and averting raised intracranial pressure and cerebral oedema. There are currently no approved neuro-specific or neurone protective medications for the management of TBI.
Improvement in morbidity and mortality from TBI has unfortunately, not been reflected in resource-poor settings like Nigeria, this is in part due to lack of facilities for resuscitation, lack of ambulance services and paramedics at site of injury, delay in transportation and prompt investigations, cost of care (out-of-pocket payment system); unavailability or insufficient space in ICU/HDU, among others.
Management of traumatic brain injury following Clinical Practice Guidelines (CPG) based on highest available evidence has been a major advance in this area, the most commonly used CPGs are adapted from publications by the Brain Trauma Foundation (BTF) [7,8,9].
Case
In 2017, a 32year old single male trader was brought into the ER by the Nigerian police following an assault on him by armed robbers. He was stabbed and thrown out of a fast-moving sedan following which he sustained a penetrating injury to the right 3rd intercostal space mid-clavicular line, lower lip laceration, and avulsion of the left upper central and lateral incisor. He reportedly bled profusely at the site of the incident. He also, sustained minor bruises to the face and an about 6cm superficial scalp laceration over the occiput.
An immediate clinical assessment was carried out which revealed a clear airway, moderate dehydration, spontaneous breathing, hypothermia 35.4oC, oxygen saturation SPO2 97%, respiratory distress and tachypnoea 40cpm, hypotension (Blood pressure 71/36mmHg) and tachycardia (pulse rate 135beats per minute). He had a GCS score of 15 (E – 4, V – 5, M – 6), he was conscious but lethargic, pupils were 3mm bilaterally and equally reactive to light. History of allergies, current medications, past medical history, last meal couldn’t be ascertained.
Resuscitation was commenced immediately at presentation with 2 litres of normal saline and 500ml of gelofusine, oxygen was administered via a facemask at 10L/m, a CTTD was passed in the 5th right intercostal space mid-axillary line, drained about 2.5 litres of sanguineous effluent, patient was catheterized and blood sample taken for urgent haemoglobin estimation, grouping, crossmatching and urgent whole blood transfusion, baseline blood chemistry, and rigid cervical collar applied. Chest radiograph and cranial CT scan were requested. Post-resuscitation, blood pressure improved to 101/65mmHg, pulse rate 121bpm.
Central Nervous system (CNS) revealed a conscious but lethargic middle-aged man, GCS score 15/15 (E – 4, V – 5, M – 6). No signs of focal neurological deficits, pupils were equally reactive to light about 3mm. Chest examination revealed breathlessness, tracheal deviation to the left, reduced chest movement and air entry over the right hemithorax, penetrating injury and skin laceration (about 3cm by 3cm) over the right 3rd intercostal space, dull percussion notes widespread over the right hemithorax. Concealed abdominal haemorrhage, and long bone, pelvic and thoracic fractures were ruled out via ultrasound scan and x-rays respectively.
Assessment of polytraumatised patient – penetrating chest injury and mild traumatic head injury was made. He was reviewed by the neurosurgical, maxillofacial surgery, anaesthetist, and physiotherapy teams.
Full blood count showed anaemia (haemoglobin 8g/dl), which was optimised with multiple whole blood transfusion, blood electrolytes and renal function were within normal limits. Lacerations were sutured and other external physical injuries were attended. Chest radiograph revealed re-expansion of the right lungs, other findings were unremarkable. CT scan was delayed for about 21 hours due to financial constraint, it revealed generalized cerebral oedema with midline shift to the left cerebral hemisphere and multiple contusions worse on the right cerebral hemisphere.
Patient’s GCS score started deteriorating about 10 hours into admission, initially to 13/15, then to 11/15 about 5 hours later. No overt signs of raised intracranial pressure were seen on examination. Measures to prevent and lower raised intracranial pressure such as nursing 30o head up, administration of 100% oxygen via non-rebreathable facemask at 10l/min were instituted.
6 hours later, patient developed tonic-clonic seizures, each episode lasting about 1 minute and aborting spontaneously. GCS score deteriorated further to 9/15, pupils became sluggishly reactive to light, he was commenced on intravenous mannitol, intravenous phenytoin and midazolam – for breakthrough seizures, and preparations made for endotracheal intubation and transfer to intensive care unit.
12 hours into ICU admission, the GCS score deteriorated to 5T/15 while in the ICU and on mechanical ventilation. He developed persistent hypotension and was commenced on inotropic support. The working diagnosis was revised to poor clinical status in a polytraumatized patient, and his relatives were counselled on the prognosis. There were no resources for arterial blood gases and other invasive monitoring measures.
24 hours later, the GCS score was found to be 2T/15 (E-1, V-T, M-1), pupils 5mm bilaterally, fixed and non-reactive to light, corneal and gag reflexes were absent, vestibulocochlear reflexes was negative. An assessment of brainstem death was made, the relatives were informed and a joint decision to discontinue care was reached. The relatives did not consent to an autopsy.
Discussion
The brain oxygen reserve lasts for only a couple of minutes and the injured brain can only tolerate severe hypoxia for a very limited period of time. Initial intervention begins at the scene of the event or in the emergency room. Immediate goals of TBI resuscitation is centred on airway protection, adequate oxygenation, and restoration of cerebral perfusion [1,2]. The index patient was initially resuscitated and stabilized in the emergency room before transfer to the intensive care unit on account of worsening neurological status. However, invasive blood pressure monitoring and cerebral perfusion pressure monitoring were not done due to unavailability.
The management of TBI is multidisciplinary as seen in index patient who was co-managed by the cardiothoracic surgery, neurosurgery, anaesthetist, nursing, physiotherapy, nutritionist and other relevant teams.
Cervical spine injury is always assumed until ruled out via imaging (cervical radiograph or computed tomography scan). Patients with mild TBI may be managed on an out-patient basis in the absence of other serious injuries, stable vitals, and normal secondary survey. The index patient was polytraumatized with concomitant traumatic brain injury. C-spine injury was assumed and rigid cervical collar applied, even though this was not ruled out via imaging throughout the duration of admission until the patient’s demise.
Neurological examination has been found to be the best clinical tool for evaluating patients with TBI. This is carried out before the administration of analgesics, sedatives or paralytic agents. Radiographic and serologic investigation provide additional information [1]. Neuroimaging (CT scan) is very useful in providing information in assessing TBI. Several neurological examinations were carried out in the index patient, and together with clinical and vital signs, were the major indicators of clinical state and intracranial pressure employed. Cranial CT scan although delayed due to financial constraints was done in the index patient on account of deteriorating GCS score which revealed cerebral abnormalities.
Intensive care management involves close neurological and physiological monitoring for all patients with moderate to severe TBI. The peak period of deterioration following TBI is within three (3) – four (4) days post-injury. That of cerebral oedema is 48 – 96 hours following injury (2 – 3 days) [2,4,7]. The common reason being the development of traumatic intracranial haemorrhage which often occurs within 9 hours from injury. The index patient sustained concomitant severe injuries and blood loss that may have led to significant secondary brain insult. He experienced progressive neurological and eventually, clinical deterioration within 72 hours of admission culminating in his unfortunate death. This is consistent with the peak period within which deterioration ensues following TBI.
Once the severely head-injured patient has been transferred to the ICU, the management is centred around the institution of high quality neuroprotection – various strategies aimed at maintaining homeostasis – stabilization of the patient, if still unstable; prevention of intracranial hypertension (ICH); maintenance of an adequate and stable cerebral perfusion pressure (CPP); avoidance of systemic, secondary brain insults; optimization of cerebral haemodynamic and oxygenation [2,4,7,8].
The index patient while in the ICU received interventions aimed at maintenance of vital functions, however, ICP and CPP were not monitored due to unavailability, as with most centres in the developing countries, especially public hospitals – mostly due to the high cost of equipment, lack of expertise, and the high level of monitoring required following the procedure.
The key goals of management of moderate to severe TBI comprise: maintenance of ICP less than 20mmHg, cerebral perfusion pressure >60mmHg, SBP >90mmHg, PaO2 >60mmHg or oxygen saturation >90%, monitoring, prevention and prompt treatment of electrolyte imbalance and acidosis, head of bed elevation – 30o to the horizontal, maintenance of normothermia, artificial airway for GCS score <8, and avoidance of steroid and prophylactic anticonvulsant medications in the first 7days [1,2,4,8].
Sustained elevated intracranial pressure above 25mmHg is an independent determinant of poor neurological outcome, and hypotension (SBP <90mmHg) carries a fourfold increase in mortality [4,8,9].
Data from SAFE study showed that colloids are contraindicated in traumatic brain injury [4,7,8,9]. Crystalloids on the other hand, are widely accepted in resuscitation. However, blood products are preferred in cases of haemorrhagic shock. The risk of infection among other complications associated with invasive ICP monitor placement is of concern to neurosurgeons and physicians. Nutritional support, glycaemic control and deep venous thrombosis prophylaxis are all vital supportive management that improves patient’s overall wellbeing.
In general, traumatic brain injury carries a significant mortality rate, and morbidity following this condition can be devastating as individuals have been seen to have residual neurological deficits up to 1 year following mild TBI. The number increases with the degree of brain injury. High quality care and aggressive efforts at maintaining homeostasis and physiologic neurological milieu – prevention of secondary brain injury, is paramount to recovery and as much return as possible to premorbid state.
Ubiquitous use of Glasgow coma scale, although have been seen to be a good predictor of outcome, may not always be accurate – intracranial pathologies may be responsible for GCS scores of less than 8. The dynamic nature of head injury and lack of collaborative studies are other contributing factors to this challenge.
The management of severe TBI requires aggressive and proactive treatment protocols with invasive monitoring (minimum an ICP bolt and neuroprotection) for the best chances at a good outcome. Unfortunately, these measures are unavailable in LMIC and is reflected in the mortality burden from TBI as highlighted in index case. The management in these countries are reactive and rely largely on clinical signs which lag significantly behind changes in the traumatised brain’s physiological milieu resulting in secondary brain injury and poor outcomes.
There is the need for standardised protocols (using the highest evidence from randomised controlled trials) in the management of traumatic brain injury in hospitals and institutions, especially in LMIC countries – where there is poor funding for healthcare systems development and infrastructure, inefficient and ineffective health insurance services, and predominant out-of-pocket payment, based on the studies conducted within the resource constraints and proactive to mitigate secondary brain injury before its onset. Increasing evidence have shown that some practices may, as a matter of fact, be associated with poor clinical and functional outcomes following TBI. This need is even more emphasized in resource-poor countries, like Nigeria where invasive monitoring is largely unavailable and the management decisions rely majorly on physicians’ clinical acumen and experience.
References
- Girling K. Management of head injury in the intensive-care unit: Table 1. Continuing Education in Anaesthesia, Critical Care & Pain, 2004; 4(2); 52–56.
- Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 2012; 20: 12.
- Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. BJA: British Journal of Anaesthesia, 2007; 99(1): 32–42.
- Sadaka FMT, Lakshmanan R, Palagiri A. Management of Traumatic Brain Injury in the Intensive Care Unit. In Traumatic Brain Injury. InTech, 2014.
- Bullock R, Chestnut RM, Clifton G, Ghajar J, Marion DW, Narayan RK, et al: Guidelines for the Management of Severe Traumatic Injury. J Neurotrauma 2000;17: 453-553.
- Eker C, Asgeirsson B, Grande PO, Schalen W, Nordstrom CH. Improved outcome after severe head injury with a new therapy based on principles for brain volume regulation and preserved microcirculation. Crit Care Med, 1998; 26: 1881-1886.
- Carney N, Totten AM, Hawryluk GWJ, Bell MJ, Bratton SL, Chesnut R, et al. Guidelines for the Management of Severe Traumatic Brain Injury 4th Edition, 2016.
- Hesdorffer D, Ghajar J, Iacono L. Predictors of compliance with the evidence-based guidelines for traumatic brain injury care: A survey of United States trauma centers. J Trauma, 2002; 52: 1202-1209.
- Marshall S, Ling G (n.d.). Critical Care Management of Traumatic Brain Injury.
- Brain_Trauma_Foundation. The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Nutrition. J Neurotrauma 2000; 17: 539–547.
- Bullock R, et al: Guidelines for the Management of Severe Traumatic Brain Injury. J Neurotrauma, 2007, 24(Suppl 1): S1-S106.
- Management and Prognosis of Severe Traumatic Brain Injury. New York: Brain Trauma Foundation and the American Association of Neurological Surgeons, 2000.
- Saul TG, Ducker TB: Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg, 1982; 56: 498-503.
- Vukic M, Negovetic L, Kovac D, Ghajar J, Glavic Z, Gopcevic A. The effect of implementation of guidelines for the management of severe head injury on patient treatment and outcome. Acta Neurochir (Wien), 1999; 141(11): 1203-1208.

