A Case of Young Male Osteoporosis Secondary to Hyperprolactinemia
Haiying Chen, Shengkai Ye*, Biying Zhang, Hailu Xing
Department of Endocrinology, the 967th Hospital of P.L.A, China
Received Date: 28/04/2022; Published Date: 10/05/2022
*Corresponding author: Shengkai Ye, Department of Endocrinology, the 967th Hospital of P.L.A, China
Abstract
This case reported a 34-year-old male with osteoporosis due to hyperprolactinemia. A 34-year-old male was admitted to the hospital due to long-term low back pain, and bone density examination indicated osteoporosis. Further examination showed that his prolactin level was significantly increased, suggesting that hyperprolactinemia led to his osteoporosis, thereby causing low back pain. On the basis of bromocriptine, calcium, vitamin D3 and bisphosphonate were given to the patient, and the pain symptoms and bone density of the patient improved rapidly. This case suggested that screening for secondary factors should be strengthened for osteoporosis in young men, and attention should be paid to the treatment of primary disease while anti-osteoporosis drug treatment.
Keywords: Hyperprolactinemia; Bone mineral density; Secondary osteoporosis
Case Report
A 34-year-old non-smoking man entered the endocrinology department of our hospital because of "intermittent low back pain for more than 2 years, aggravated for 1 week." Two years before admission, there was intermittent low back pain, which was aggravated after standing for a long time. At that time, the double-energy X-ray bone mineral density of the hip joint showed mild bone loss, but he didn't pay attention, just take calcium and vitamin D3 intermittently. One week before admission, the pain in low back was aggravated, and load bearing capacity decreased significantly. He was admitted for further diagnosis and treatment. The patient was diagnosed with chronic atrophic gastritis with bile reflux and chronic colitis 10 years ago, and had been treated with mesalamine. Currently, he has no symptoms of discomfort, and his diet and stool are normal.
Physical examination of the heart, lungs and abdomen showed no abnormality. There was no fixed tenderness in the spine, no local redness, fever and swelling. Normal muscle strength and muscle tension in the limbs, and no pathological signs were extracted. Blood routine and biochemical tests including full blood count, erythrocyte sedimentation rate, hsCRP, serum calcium, serum magnesium, serum phosphorus, liver and kidney function were normal. Immunologic test including rheumatic immunity related antibodies and serum immunoglobulin is normal were normal. Serum protein electrophoresis was normal and monoclonal immunoglobulin was not observed. Relevant hormone detection indicated 25- hydroxy vitamin D was decreased, parathyroid hormone, calcitonin, and thyroid function were normal, prolactin was increased, and estradiol was slightly decreased. Urine Bence-Jones protein was negative, urine routine was normal and urinary calcium excretion was increased (Table 1). Both parathyroid ultrasonography and renal ultrasonography were normal. Dual-emission X-ray Absorptiometry bone mineral density examination showed osteoporosis (Table 2, Figure 1). Therefore, osteoporosis is clearly diagnosed.
This patient was a young male, so we considered his osteoporosis as secondary osteoporosis. Many diseases can lead to secondary osteoporosis. Biochemical examination and relevant antibody examination of the patient were normal. Therefore, we excluded osteoporosis caused by rheumatic immune disease, blood system disease and kidney disease. The patient had a history of colitis, but the calcium absorption is mainly in the upper part of the small intestine, and after treatment, the patient had no digestive symptoms, so the patient does not have calcium absorption disorder and intestinal over secretion. This patient is deficient in vitamin D, but vitamin D deficiency (10-20 ng/mL) or insufficiency (20-30 ng/mL) is a universal problem in the world. The intake of vitamin D in the population of China is significantly lower than the nutritional standard. Considering the age of the patient and comparisons with peers, osteoporosis in this patient cannot be fully explained by vitamin D deficiency. Hormone assay indicated hyperprolactinemia, so it was confirmed that osteoporosis in this patient was caused by hyperprolactinemia.
Patients were given oral administration of bromocriptine at 2.5mg per day, 1 bag of calcium carbonate D3 granules per day (each bag contained 500mg calcium and 200IU vitamin D3), 2.5ug calcitriol per day, and 100ml (5mg) of zoledronic acid injection for intravenous infusion once. The review of prolactin was decreased slightly after two months, but still higher than normal (Table 1), so the amount of bromocriptine was increased to 5mg per day. The re-examined bone mineral density showed a significant improvement in bone mineral density (Table 3, Figure 2), but Vitamin D levels remained low (Table 1). Patients were instructed to continue to take calcium carbonate D3 granules orally for 1 bag per day and calcitriol 2.5ug per day.
Table 1: Laboratory data.
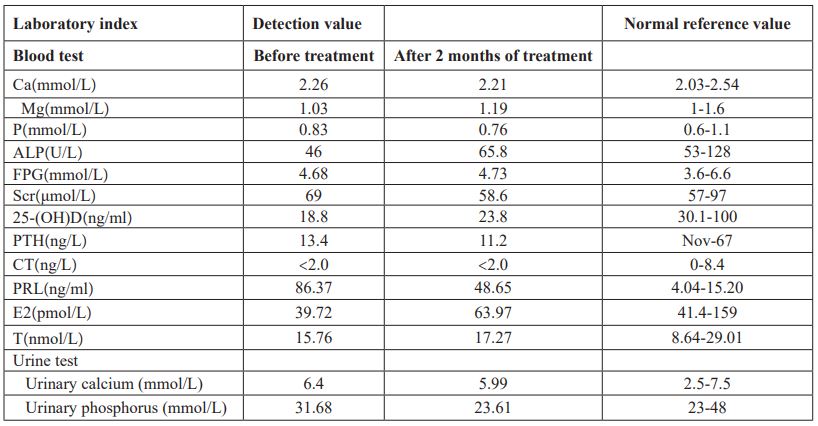
Table 2: Data of BMD of the left hip and the lumbar spine before treatment.
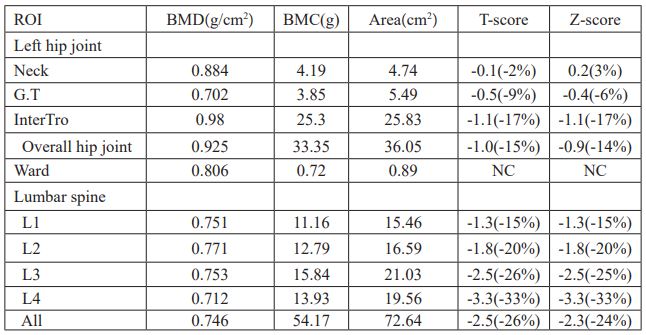
Table 3: Data of BMD of the left hip and the lumbar spine after treatment.
Discussion
Osteoporosis(OP) is a systemic bone metabolic disease in which the microstructure of bone tissue is destroyed, bone is thinned, bone mineral composition and trabecular bone number are reduced, bone density is reduced, and bone fragility is increased [2]. China is the country with the largest absolute number of elderly people in the world. As the population aging, the population of OP and bone loss is increasing year by year, and the incidence of osteoporotic fractures is also increasing. The medical and nursing care of the OP and its fractures requires a lot of manpower, material resources and financial resources, resulting in a heavy family and social burden [3], which has become a serious public health problem in China and the world. What is even more worrying is that although the recognized elderly population is a high-risk population of OP, such diseases can be seen in all ages, and the incidence of OP and osteoporotic fractures in young people is also significantly increased. The pathogenesis of OP is complex. Current research suggests that endocrine factors, genetic factors, nutritional status, physical factors, lifestyle and psychological status are closely related to the occurrence of OP.
Secondary OP is mainly caused by endocrine and metabolic diseases, rheumatic immune diseases, blood system diseases, kidney diseases, drugs or long-term brakes. Secondary OP occurs mainly in people who are not considered to be at high risk for osteoporosis, and is therefore easily missed and misdiagnosed, especially in men and premenopausal women. It has been confirmed that approximately 40% of male osteoporosis is due to secondary factors [1]. Prolactinoma and hyperprolactinemia are linked with osteoporosis [4-5], but there are few reports on male patients in this area. Here, we presented a case of male OP caused by hyperprolactinemia. The patient had a history of colitis, but the absorption of calcium is mainly in the upper part of the small intestine, and the patient had no gastrointestinal symptoms after treatment, so the patient did not have calcium absorption disorder and excessive intestinal secretion. Biochemical tests and related antibody tests were normal, and so OP caused by rheumatic immune diseases, blood system diseases, and kidney diseases were excluded. Further examination revealed hyperprolactinemia; thereby confirming that osteoporosis in this patient was caused by hyperprolactinemia.
Hyperprolactinemia is closely related to bone metabolism. In patients with hyperprolactinemia, there was a significant reduction in bone density, with reduced lumbar spine and femoral neck bone mass, or osteoporosis [6-9]. Most studies have found that bone mineral density values were negatively correlated with prolactin levels and disease duration [6-8]. While Greenspan SL et al [9] found that cortical osteopenia was significantly related to the duration of hyperprolactinemia but not to the absolute levels of prolactin.
It is currently believed that hyperprolactinemia causes OP through a variety of mechanisms. Hyperprolactinemia inhibits the gland axis, causing changes in estrogen levels, leading to bone development and abnormal calcium and phosphorus metabolism. Naliato EC et al. [8] found significant associations between BMD and PRL, testosterone and estradiol, and estradiol was the main determinant of BMD. Our patient had low levels of estradiol before treatment, which may have contributed to his osteoporosis. It is also believed that high prolactin can cause reduced bone mass by decreasing Osteocalcin (OC) concentration, a specific index of bone formation. OC concentrations were significantly lower in female patients with microprolactinoma than in controls. Serum OC levels significantly increased after dopaminergic agents’ treatment [10].
Treatment of secondary OP includes the treatment of primary disease and osteoporosis. If the condition allows, the two can be performed simultaneously. Studies have shown that the use of different dopamine agonists to reduce prolactin levels can increase bone mineral density slightly, but cannot restore bone mass, suggesting that reducing prolactin levels can prevent additional bone loss [6,7]. Therefore, we gave this patient bromocriptine 2.5mg/day orally to reduce prolactin levels. After 2 months of application, the prolactin was decreased, but it was still higher than normal, so bromocriptine was increased to 5mg/ day.
The treatment of OP itself mainly aims at restoring abnormal bone metabolism and remodeling bone health through drug treatment while correcting risk factors. In this case, the patient was treated with anti-osteoporosis drugs including calcium, vitamin D and bisphosphonate while actively reducing serum prolactin. After treatment, the patient's bone mineral density and clinical symptoms have been improved.
In conclusion, this case report indicates that after the diagnosis of OP, the screening of secondary factors should be strengthened to avoid missed diagnosis and misdiagnosis. Once the secondary OP is diagnosed, the corresponding prevention and treatment measures should be initiated immediately. The anti-osteoporosis drugs can be added on the basis of active treatment of the primary disease, which can quickly improve bone health and clinical symptoms.
References
- Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev, 2008; 29(4): 441-464.
- Ahmed SF, Elmantaser M. Secondary osteoporosis. Endocrine Development, 2009; 16: 170–190.
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int, 2014; 25(10): 2359-2381
- Koloszár S, Gellén J, Kovács L. The value of plasma prolactin level determination in the diagnosis of postmenopausal osteoporosis. Orv Hetil, 1997; 138(2): 71-73.
- Shibli-Rahhal A, Schlechte J. The effects of hyperprolactinemia on bone and fat. Pituitary, 2009; 12(2): 96-104.
- Di Somma C1, Colao A, Di Sarno A, Klain M, Landi ML, Facciolli G, et al. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J Clin Endocrinol Metab, 1998; 83(3): 807-813.
- Colao A, Di Somma C, Loche S, Di Sarno A, Klain M, Pivonello R, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf), 2000; 52(3): 319-327.
- Naliato EC, Farias ML, Braucks GR, Costa FS, Zylberberg D, Violante AH. Prevalence of osteopenia in men with prolactinoma. J Endocrinol Invest, 2005; 28(1): 12-17.
- Greenspan SL, Neer RM, Ridgway EC, Klibanski A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med, 1986; 104(6): 777-782.
- Sartorio A, Conti A, Ambrosi B, Muratori M, Morabito F, Faglia G. Osteocalcin levels in patients with microprolactinoma before and during medical treatment. J Endocrinol Invest, 1990; 13(5): 419-422.

