SARS-CoV2 Cholangiopathy
Georgia Chichelero, Natália Boniatti, Renata Asnis Schuchmann, Simone Magagnin Wajner*
Internal Medicine Division, Hospital de Clínicas de Porto Alegre, Brazil
Internal Medicine Department, Universidade Federal do Rio Grande do Sul, Brazil
Hospital De Clínicas de Porto Alegre, Porto Alegre, Brazil
Received Date: 05/02/2022; Published Date: 16/02/2022
*Corresponding author: Dr. Simone Magagnin Wajner, Internal Medicine Division, Hospital de Clínicas de Porto Alegre, Rua Ramiro Barcelos, 2350, CEP 90035-003 – Porto Alegre, RS, Brasil.
Introduction
Coronavirus pandemic has been a steady challenge for doctors and healthcare professionals. Beyond the respiratory viral injury, a multisystemic involvement associated with SARS-COV2 infection is observed, including hepatic impairment. Liver enzymes elevation is a frequent finding during the course of the disease and still has uncertain prognostic value. Meanwhile, cholestasis biomarkers increment is not as usual and can be attributed to SARS-CoV2 cholangiopathy once associated with radiologic findings as well as appropriate time period.
Given the high prevalence of coronavirus infection and the scarce evidence about SARS-CoV2 cholangiopathy, a better understanding of this specific viral injury is of paramount importance. We hereby present a clinical case assisted at Hospital de Clínicas de Porto Alegre between march and may of 2021.
Case Report
We present a previously healthy, 36 years-old male patients. SARS-COV2 infection evolved within two days to respiratory failure due to hypoxia and therefore acute respiratory distress syndrome. He then required invasive ventilatory support and prone maneuver owing to refractory hypoxia as well as a 10-day glucocorticoid course. He consequently developed multiple infections, most of them by multidrug resistant pathogens, leading to a wide spectrum of antimicrobial therapy exposition. During follow up the subject also presented other complications as acute non-biliary pancreatitis and pulmonary embolism.
In the fourth month of hospitalization, jaundice and altered liver enzymes occurred. Laboratory findings included expressive elevation of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin at the expense of direct fraction (Table 1). The possibility of pharmacologic hepatotoxicity related to acetaminophen or antimicrobials, as well as hepatic injury after hemodynamic shock and/or uncontrolled infection or even direct SARS-CoV2 liver damage were considered. Other viruses’ infection was excluded. Ultrasonographic evaluation identified mild biliary tract enlargement. Progressive deterioration of liver biomarkers led to cholangiorresonance imaging (Figure 1). The study confirmed irregular dilation of biliary intrahepatic tract with small stenotic areas bordering short distended zones consisted with critical care patient secondary to sclerosing cholangitis. Treatment with ursodeoxycholic acid was initiated and liver biopsy proposed to enlighten the clinical findings. Pathologyc analysis (Figure 2) revealed moderate ductular reaction associated with mixed inflammatory infiltrate and exuberant cholestasis which endorsed the radiologic findings and suggested post-COVID19 cholangiopathy. Cholestatic biomarkers were stabilized after the introduction of ursodeoxycholic acid. Patient was considered to liver transplant, but the procedure was not necessary. Hospital discharge was possible after 167 days with outpatient follow.
Table 1: Laboratory follow-up until discharge.
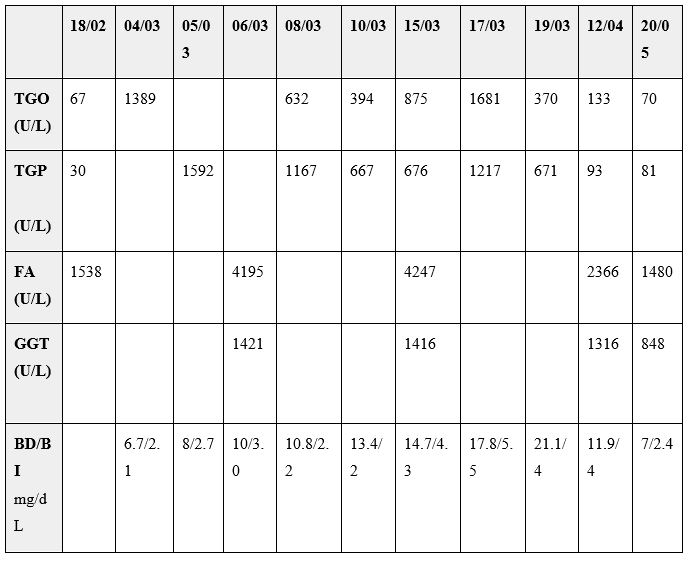
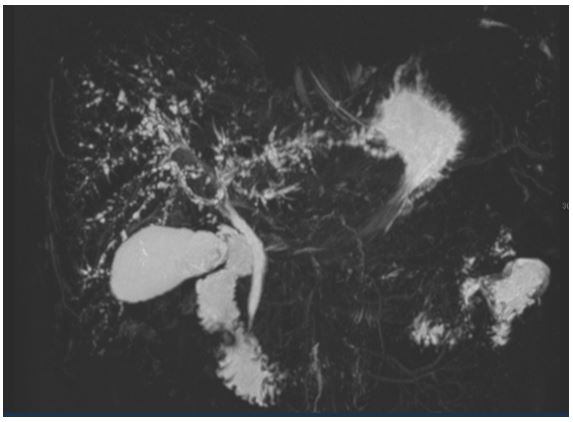
Figure 1: Cholangioresonance imaging: irregular dilation of biliary intrahepatic tract with small stenotic areas bordering short distended zones consisted with critical care patient secondary to sclerosing cholangitis.
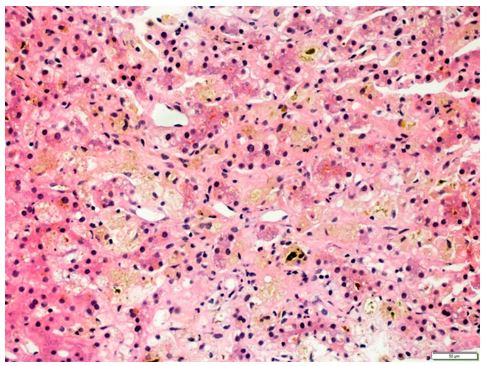
Figure 2: Moderate ductular reaction associated with mixed inflammatory infiltrate and exuberant cholestasis.
Discussion
The management of respiratory distress syndrome has become a routine for healthcare workers during the COVID-19 pandemic. Beyond classic pulmonary damage, the virus has multisystemic potential involvement, including liver and bile ducts as detailed above. COVID-19 associated liver injury is defined by impairment of hepatic function during the progression of the viral infection regardless of the presence of previous hepatic disease. Estimated incidence of altered liver enzymes in hospitalized patients is 14 to 53%, typically more frequent in male patients [1,2]. Some advocate that no specific patterns of liver disorder prove direct correlation with de SARS-CoV2 infection [3], as the impairment might be secondary to tissue hypoxia, inflammatory hyperactivation, drug toxicity or baseline hepatic disorder [2,4,5]. On the other hand, there is increasing evidence about characteristic patterns in the coronavirus related liver disease [6]. Direct hepatocyte injury is still not fully comprehended but an association with the angiotensin converting enzyme (ACE2) in the hepatocyte could be part of the physiopathology mechanism [6].
Liver tissue expresses ACE2 in comparable levels as the type 2 pneumocytes explaining the potential viral tropism for this organ [4]. Within the liver, ACE2 receptors are expressed in 59% of the biliary tract cells and in 2% of the hepatocytes [2]. It is noteworthy that this proportion does not find correspondence in the more frequent pattern of altered liver enzymes in COVID-19 patients - most of them experience more intense levels of ALT/AST than bilirubin or alkaline phosphatase/gamma glutamyl transferase (FA/GGT), suggesting that maybe more than one mechanism can be involved [4,5]. Likewise, the direct hepatocyte damage itself, a biliary duct targeted viral injury has been described recently, named "post-COVID-19 cholangiopathy". Although not defined as a specific pattern of virus involvement we aim to endorse it with this clinical case report. Previous data support that meanwhile the hepatocyte injury is transient and rapidly resolved, the cholangiocyte is of significant morbidity over time with consequent worst global prognosis [4,7]. Liver transplant may be considered in cases of persistent jaundice, liver failure or recurrent bacterial cholangitis [8,9]. The pathologic findings also have incipient data available. Nonspecific findings are generally described as microvesicular steatosis or mild lobular and portal activity. Intracellular viral inclusion might be involved [10].
Conclusion
We presented a previous healthy patient evolving to severe coronavirus infection along with multisystemic complications but exuberant hepatic impairment above all. Particular imaging and histology findings reassure a prevailing bile ducts involvement. Undoubtedly better elucidation about the COVID-19 cholangiopathy is mandatory including its physiopathology, natural history and management. As we comprehend this novel SARS-CoV2 infection feature we magnify our ability to recognize and treat it.
Competing Interests: Authors declare no conflict of interest.
GRANT INFORMATION
The author(s) received no specific funding for this work.
References
- Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int, 2020; 40(6): 1278-1281. doi: 10.1111/liv.14470. PMID: 32251539.
- Kullar R, Patel AP, Saab S. Hepatic Injury in Patients With COVID-19. J Clin Gastroenterol, 2020; 54(10): 841-849. doi: 10.1097/MCG.0000000000001432. PMID: 32976196.
- Schmit G, Lelotte J, Vanhaebost J, Horsmans Y, Van Bockstal M, Baldin P. The Liver in COVID-19-Related Death: Protagonist or Innocent Bystander? Pathobiology, 2021; 88(1): 88-94. doi: 10.1159/000512008. Epub 2020 Oct 27. PMID: 33108789; PMCID: PMC7705929.
- Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020; 73(5): 1231-1240. doi: 10.1016/j.jhep.2020.06.006. Epub 2020 Jun 15. PMID: 32553666; PMCID: PMC7295524.
- Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, et al. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis, 2020; 40(3): 321-330. doi: 10.1055/s-0040-1715108. Epub 2020 Sep 4. PMID: 32886936.
- Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021; 56(3): 218-230. doi: 10.1007/s00535-021-01760-9. Epub 2021 Feb 1. PMID: 33527211; PMCID: PMC7849620.
- Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, et al. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021; 116(5): 1077-1082. doi: 10.14309/ajg.0000000000001154. PMID: 33464757.
- Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, et al. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol, 2021; 116(7): 1414-1425. doi: 10.14309/ajg.0000000000001264. PMID: 33993134.
- Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, et al. Post-Covid-19 Cholangiopathy-A New Indication for Liver Transplantation: A Case Report. Transplant Proc, 2021; 53(4): 1132-1137. doi: 10.1016/j.transproceed.2021.03.007. Epub 2021 Mar 12. PMID: 33846012; PMCID: PMC7953456.
- Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther, 2020; 52(2): 267-275. doi: 10.1111/apt.15813. Epub 2020 Jun 2. PMID: 32402090; PMCID: PMC7272838.

