Fibrosing Cholestatic Hepatitis in Renal Transplant Recipient with Hepatitis B Core Total Positive Antibody
Arshad Ali Jariko, Hina Ismail, Raja Taha Yaseen Khan*, Zain Majid, Arz Muhammad, Dilnawaz Samoon, Zahid Shah, Kiran Bajaj and Nasir Hasan Luck
Department of Hepatogastroenterology, Sindh Institute of Urology and Transplantation
Received Date: 26/01/2022; Published Date: 10/02/2022
*Corresponding author: Raja Taha Yaseen Khan, Department of Hepatogastroenterology, Sindh Institute of Urology and Transplantation, Iraq
Abstract
Fibrosing cholestatic Hepatitis (FCH) is a fatal manifestation of HBV and HCV infection in liver allograft recipients associated with detrimental outcomes. However, the incidence of FCH is rising in non-solid organ transplant recipients. Here, we present to you a case of forty-six years old male who was HBV total positive and underwent renal transplantation, was evaluated for deranged liver enzymes and underwent liver parenchymal biopsy with findings suggestive of FCH.
Keywords: Fibrosing cholestatic hepatitis; Renal Transplantation
Introduction
Fibrosing cholestatic hepatitis (FCH) has known to be a fatal manifestation of hepatitis B virus (HBV) infection in liver allograft recipients described as rapid and progressive deterioration in graft functions, manifested by severe jaundice, coagulopathy, encephalopathy and death within 4-6 weeks of onset. There is derangement of laboratory parameters with elevated serum bilirubin level, prolonged prothrombin time, and transaminases. Previously reported cases were all associated with detrimental outcomes, and high levels of expression of viral antigen in the liver. The histopathologic changes of FCH are marked by hepatocyte ballooning (swelling), intracellular and canalicular cholestasis, and periportal and/or perisinusoidal collagen deposition.
Case Report
Forty-six-year-old male, with history of end stage renal disease underwent live related Renal transplant 11 years back with past history not significant for any chronic illness apart from right orchiectomy for undescended testis. Post-transplant, his renal functions remained stable for more than 10 years.
His was on maintenance immunosuppression Azathioprine (AZA), Methylprednisolone (MP) and cyclosporine (CsA). Currently he was admitted with complaints of high-grade fever for 20 days, documented up to 102°F, associated with rigors and chills, mostly occurring at night and relieved by taking oral paracetamol (1g /day). He also had complained yellow discoloration of sclera for 5 days, which was noticed by family members, was sudden in onset, progressive in nature, not associated with itching, clay color stool or dark color urine. Patient denied history of vomiting, loose stools, hematemesis, melena or bleeding PR.
On admission, liver enzymes were derranged with total bilirubin of 8.36mg/dl and direct billirubin 5.03mg/dl, alanine aminotransferase [ALT] of 104 IU/L, aspartate aminotransferase [AST] of 83 IU/L, Ɣ-glutamyl transferase (GGT) of 55 mg/dl IU/L and alkaline phosphatase [ALP] of 288IU/L. Serology markers for HCV and cytomegalovirus (CMV) were negative. Three days later, GGT levels increased to 145 IU/L and ALP levels to 310 IU/. Rise in total bilirubin was also noted from 8.36 to 20.35 mg/dL, ALT to 167 IU/L; and AST to 88 IU/L. An ultrasound liver examination did not show any changes. Antibodies against HAV IgM, HEV igM viruses were undetectable, HCV RNA was negative. Antibodies to Hepatits B core total came out to be positive. Autoimmune serology and IgG were also inconclusive. Any hepatotoxic drug including Azathiopurine was withdrawn (due to high incidence of sinusoidal obstruction syndrome in this population). During the course of hospital admission, liver functions further deteriorated. Three weeks into hospital admission, values for GGT were 519 IU/L; ALP IU/L; ALT 9 IU/L; AST, 203 IU/L; and bilirubin was 37 mg/dL. Liver biopsy was performed with histological changes persistent with the diagnosis of FCH.
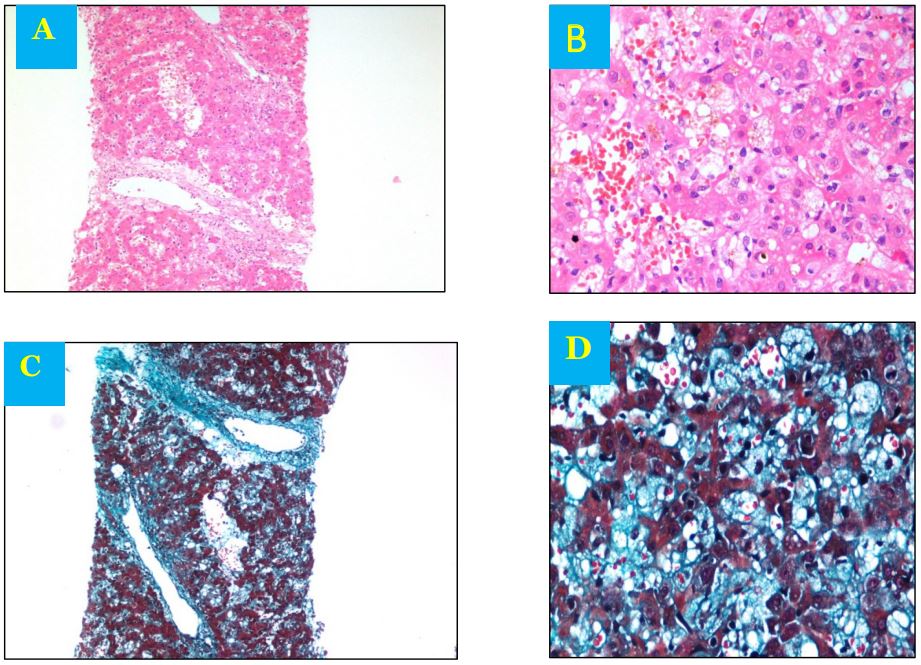
Figure 1: A- Effaced architecture with focal hepatocyte dropout Dilated and congested sinusoidal spaces Portal tracts are expanded with inflammatory cells and fibrosis B- Dilated and congested sinusoidal spaces with intracanalicular and intracellular cholestasis C- Trichome stain showing portal tract fibrosis D-Trichome stain showing sinusoidal fibrosis.
Discussion
FCH is a progressive and fatal form of viral hepatitis that can affect immunocompromised patients. It has been reported in organ transplant recepients, hepatitis B virus (HBV), hepatitis C virus (HCV) [1] human immunodeficiency infection (HIV) and cytomegalovirus infection (CMV) [2]. It is frequently seen after liver transplantation; few cases are also reported as rare complication of renal transplantation [3-5]. FCH is characterized by rapid progression to liver failure manifested by jaundice,particularly with increasing bilirubin and raised aminotransferases [6],coagulopathy and encephalopathy, and it can lead to death within weeks [6]. The diagnosis of FCH is based on histology showing marked hepatocyte ballooning, cholestasis, and extensive periportal and/or perisinusoidal fibrosis. There is minimal infiltration of inflammatory cells with an extensive periportal ductal reaction, cholestatsis and intense positivity for HBs and HBc antigens in an immunocompromised situation7. Hepatocellular damaged seen in immunocompromised patients is secondary to cytopathic effect of increased viral load inducing hepatocyte apoptosis, cellular degeneration and necrosis [6].
In our patient, histopathology report depicted mild to moderate expansion with lymphocytic infiltrate and fibrosis.Lobular parenchyma showed sinusoidal dilatation and congestion.Foci of confluent necrosis with focal drop out.Foci of intracanalicular and intracellular cholestasis noted.Focal ductular and portal and perisinusoidal fibrosis also noted.Serology showed positive hepatitis B core antigen.
Conclusion
Our report indicates that FCH can occur not only in orthotopic liver transplant recipients but also in other immunosuppressed patients such as HB core total antibody positive patients undergoing non liver solid organ transplantation. There is a need of understanding the mechanism of FCH and formulation of a better therapeutic strategy. Finally, FCH should be kept in mind in managing patients with progressive hepatic failure and deranged liver enzymes post solid organ transplantation.
References
- Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology, 1996; 23: 971–976.
- Agarwal SK, Kalra V, Dinda A, et al. Fibrosing cholestatic hepatitis in renal transplant recipient with CMV infection: a case report. Int Urol Nephrol, 2004; 36: 433–4352
- Lam PW, Wachs ME, Somberg KA, et al. Fibrosing cholestatic hepatitis in renal transplant recipients. Transplantation, 1996; 61: 378–381
- Zylberberg H, Carnot F, Mamzer MF, et al. Hepatitis C virus-related fibrosing cholestatic hepatitis after renal transplantation. Transplantation, 1997; 63: 158–160.
- Toth CM, Pascual M, Chung RT, et al. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation, 1998; 66: 1254–1258.
- Lee HK, Yoon GS, Min KS, Jung YW, Lee YS, Suh DJ, et al. Fibrosing cholestatic hepatitis: a report of three cases. Int J Clin Exp Pathol, 2000; 15: 111-114.
- Xiao SY, Lu L, Wang HL. Fibrosing cholestatic hepatitis: clinicopathologic spectrum, diagnosis and pathogenesis. Int J Clin Exp Pathol, 2008; 1: 396-402.

