A Real-World User Survey Study Conducted with a Hypertonic Seawater Nasal rrigation Solution Comprising Algal and Herbal Ingredients in Patients with Ent Disorders
Stella Georgiou, Konstantinos Alevizopoulos*
Research & Development Department, Gerolymatos International S.A., Greece
Received Date: 12/01/2022; Published Date: 25/01/2022
*Corresponding author: Konstantinos Alevizopoulos PhD, 13 Asklipiou Str. 14568, Kryoneri, Attica, Greece
Abstract
Limited real-world data exist on the clinical benefits of medical devices for cleansing and decongesting the nasal mucosa. To that end, a user survey study with a hypertonic seawater nasal irrigation solution comprising algal and herbal ingredients (HSS-Plus) was conducted in patients with ENT disorders. One hundred patients who experienced otorhinolaryngological (ENT) symptoms were recruited in private outpatient settings. Patients were advised to perform nasal irrigations with this medical device according to the products’ instructions for use over a period of up to two weeks. At the end of the evaluation period, scores of symptom improvement, nasal cleansing, duration of symptoms and total effectiveness, safety and ease of product use were recorded in questionnaires using Visual Analog Scale (VAS) scores. A high score of 8.4/10 was assigned by patients regarding the product’s ability to improve nasal cleansing. Enhanced decongestive action (score 8.2/10) and faster symptom improvement (score 8.0/10) was perceived by the product users. Patients were very satisfied with the product giving a high score of 24.7/30 on product’s total effectiveness. The medical device had a high safety profile with few minor adverse events noted. These results support adjunctive treatment of HSS-Plus for symptomatic relief in patients with ENT disorders.
Keywords: Nasal irrigation; User survey; Hypertonic solutions; Sinonasal symptoms; Herbal and algal ingredients; ENT
Introduction
Patients with infection of the nose and throat of bacterial or viral etiology such as common cold and flu can present with a variety of sinonasal symptoms. These may include congested nose or rhinorrhea, nasal and sinus pressure as well as fever, anosmia, ageusia, presence of cough, and/or purulent nasal secretions. Colds are usually self-limited and last seven to ten days, although symptoms can endure up to three weeks. Symptomatic treatment includes pharmacological medications and/or over-the-counter medicines aiming to reduce symptoms while improving the quality of life.
Nasal irrigation is recommended as an adjunctive treatment for ENT disorders. Nasal lavage with hypertonic saline/seawater was shown to have a favorable effect in the management of sinonasal conditions and symptoms [1,2]. Among different hypertonic solutions, seawater solutions of 2.3% NaCl are frequently used in adult and pediatric populations [3-5].
Additional ingredients have been introduced to sinonasal irrigation fluids to improve their action and/or provide additional ancillary benefits. These may include algal or plant extracts, essential oils, vitamins, hydrating agents or other ingredients. In the current user survey, the nasal irrigation solution is a hypertonic seawater solution (2.3% NaCl) comprising algal and herbal ingredients (HSS-Plus). Two families of additional ingredients are introduced to HSS-Plus, namely: I) algal extracts from Undaria pinnatifida and Spirulina platensis. These ingredients have been shown to possess interesting biological actions including, among others, anti-oxidant, anti-inflammatory, anti-viral and hydrating properties when administered systemically in animal models or humans [6]. Of particular interest for topical applications are barrier and hydrating properties contributed by these ingredients. Specifically, marine seaweed polysaccharides, such as fucoidan from Undaria pinnatifida, have been found to inhibit influenza, parainfluenza, avian influenza strains and herpes viruses [7,8]. Experimental results have also shown that fucoidan from Undaria pinnatifida inhibits viral entry of SARS-CoV-2 [9]. A similar mechanism of adhesion blockage has been reported for bacteria [10] arguing for a general barrier role of the ingredients against pathogens. Finally, the ingredients exhibit hydrating properties as based on their capacity to absorb moisture [11]. HSS-Plus further comprises a second family of ingredients: II) herbal ingredients from Eucalyptus globulus, Mentha spicata, and Thymus vulgaris. These ingredients also possess interesting systemic properties [12-14]. However, they are only expected to provide a refreshing olfactory sensation at low concentrations [15].
To obtain clinical data with HSS-Plus in order to evaluate its efficacy and safety, a user survey study was conducted assessing its ability to alleviate respiratory symptoms in patients with ENT disorders within the scope of routine care in real-life.
Methods
Study settings
A prospective user survey study was conducted between February 2021 and October 2021. The study included 100 patients recruited by 10 General Practitioners or Pathologists who run private practices in Athens, Greece.
Questionnaires and Sino nasal outcomes
Visual analog scales (VAS) are commonly used for the evaluation of symptoms in patients with otorhinolaryngological (ENT) disorders [16]. In this study, we have used a 10-point scale (0=strongly disagree, 10=strongly agree) for symptom assessment. The patient questionnaire consisted of questions regarding general personal information, sinonasal symptoms, safety, ease of use, effectiveness and overall product evaluation scores (Figure 1). Each questionnaire was filled at the end of the evaluation period.
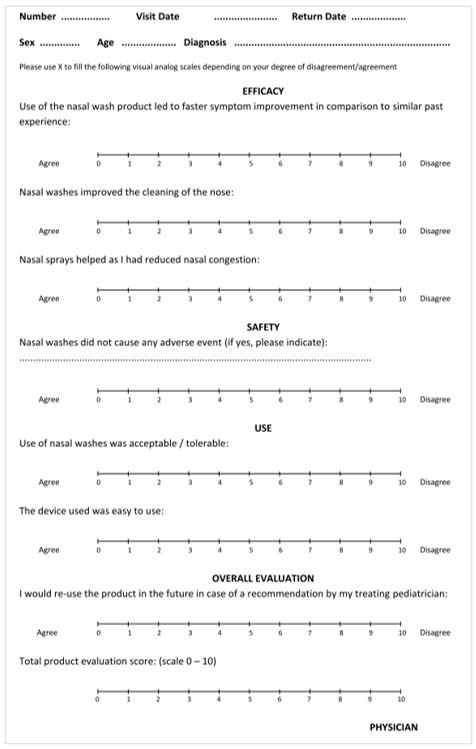
Study participants
The user survey study involved patients who experienced one or more symptoms of ENT disorders with the ability to use a nasal spray. ENT disorders could include various forms of rhinitis, common cold & flu, rhinosinusitis and allergy among others. Patients were prescribed the indicative medicated treatment according to their condition. They were further encouraged to rinse their nasal sinuses with HSS-Plus (Sinomarin® Plus Algae Cold & Flu Relief, Gerolymatos International) according to the manufacturer’s use instructions: 1-2 sprays in each nostril, 2-3 times daily for adults & children over 12 years independently of the use of medication. Age and sex were recorded. A follow-up visit was planned at least two weeks after the first visit.
Study device
The nasal spray under evaluation (HSS-Plus, 100 mL) is composed of hypertonic (2.3% NaCl) seawater solution with two algal extracts (Undaria pinnatifida and Spirulina platensis) and three herbal essential oils and extracts (essential oils from Eucalyptus globulus and Mentha spicata, and a Thymus vulgaris extract). HSS-Plus aims at relieving symptoms of the common cold & flu including nasal congestion, sinus pressure and runny nose, cleansing the nasal cavities and moisturizing the nasal mucosa.
Results
One hundred patients (average age: 48±17 years) participated in this study (43 males and 57 females). The majority (58/100) suffered from various forms of rhinitis (allergic, chronic, vasomotor, viral or other) and common cold (15/100). Six more patients with sinusitis, 5 patients with the flu, 2 patients with otitis and 2 patients with allergic disorders also participated. Rhinopharyngitis and respiratory infections were noted in one subject each. Three patients with asthma were also enrolled (Table 1).
Nasal congestion was reported in several cases (18/100); most of the times due to COVID-19 disease (10/18 cases) (Table 1). The follow-up visit occurred at 13±6 days.
Table 1: Patients’ characteristics.

Figure 1 : HSS-Plus user Survey questionnaire.
Efficacy of the nasal spray
HSS-Plus achieved a high score of 24.7/30 indicating its effectiveness in helping patients to cope with symptoms of ENT disorders. Patients gave a high score of 8.4/10 in improvement of cleaning of the nose after using the nasal spray, agreeing that the product helped reducing nasal congestion assigning a score of 8.2/10. Use of the product increased symptom-free days with score of 8.0/10 (Figure 2).

Figure 2: HSS-Plus efficacy scores.
Safety of the nasal spray
HSS-Plus was safe to use achieving a very high safety score (9.3/10). Only 4 minor adverse events were reported; 2 cases with nasal burning sensation, 1 case presenting with mid irritation and 1 case with epistaxis. These were mild and resolved spontaneously.
Use of the nasal spray
The use of HSS-Plus was highly acceptable/tolerable (score 8.8/10). The experience of patients suffering from sinonasal ENT symptoms that used it revealed that the device was easy to use (9.1/10) (Figure 3).

Figure 3: Use of HSS-Plus.
Overall evaluation use of nasal spray
The users gave a high score (8.3/10) answering whether they would re-use the product in the future in case of a recommendation by their treating physician. The total product evaluation score given by the patients was also high (8.6/10).
Discussion
Nasal irrigation is recommended as an adjunctive treatment for ENT disorders. Nasal lavage with hypertonic saline/seawater was shown to have a favorable effect in the management of sinonasal conditions [1,2]. Nasal obstruction is one of the most cumbersome symptoms. HSS-Plus nasal spray is highly effective in improving cleaning of the nose, reducing nasal congestion and achieving faster symptom resolution as indicated by high effectiveness scores. HSS-Plus nasal spray has similar properties to 2.3% NaCl hypertonic solutions in terms of ENT symptom management such as improvement of nasal obstruction/comfort and rhinorrhea in children and adults with rhinitis and acute respiratory diseases observed in several clinical studies [3-5].
The nasal spray evaluated herein contains 2.3% NaCl hypertonic seawater with Undaria pinnatifida and Spirulina platensis algal ingredients and Eucalyptus globulus, Mentha spicata, and Thymus vulgaris herbal ingredients. These ingredients exhibit interesting barrier-like and hydration properties and/or exert olfactory stimulating actions which are desirable and relevant for topical applications in respiratory conditions aiding symptomatic relief. Supplementing the irrigation solution with algal extracts and herbal essential oils and extracts probably contributed to the positive action of this medical device due to ancillary barrier-like and hydrating properties while offering at the same time a refreshing sensation.
In this user survey study conducted in real-life settings, HSS-Plus was well-tolerated by the users and had an excellent safety profile. The few minor adverse events were mild, self-limited and subsided spontaneously. This is in agreement with observations reported in the literature for other hypertonic solutions [2]. Conclusively, the risk-benefit of nasal irrigations with hypertonic solutions is highly favorable.
Ease of use of a nasal spray daily constitutes a major desirable property for these devices. So far, limited data are available regarding patient satisfaction. This real-world use survey study underscores the users’ high satisfaction with the product’s efficacy in their daily experience in ENT symptom relief. This is also underscored by the users’ willingness for future use.
Conclusion
Analysis of the user survey results obtained from HSS-Plus use in patients with ENT disorders confirmed the properties of the product. Overall, product users were highly satisfied with the product’s efficacy and safety profile. Improved Sino nasal symptom control was achieved in real-life settings. Very low incidence of adverse events suggests that it poses a minimal safety risk. These results corroborate adjunct use of this nasal spray in patients with various Sino nasal conditions.
Conflicts of Interest/ Competing Interests: No conflict of interest was declared by the authors.
Grant Information: The author(s) received no specific funding for this work.
Acknowledgements: None
References
- Principi N, Esposito S. Nasal Irrigation: An Imprecisely Defined Medical Procedure. Int J Environ Res Public Health, 2017; 14(5): 516. doi: 10.3390/ijerph14050516. PMID: 28492494; PMCID: PMC5451967.
- Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-analysis. Am J Rhinol Allergy, 2018; 32(4): 269-279. doi: 10.1177/1945892418773566. Epub 2018 May 18. PMID: 29774747.
- Gonzalez G, Sanchez AY, Mejia R. An investigational, prospective, longitudinal, comparative, multicentre, open-label study on the efficacy and tolerability of Sinomarin Spray for the treatment of rhinitis; J Fed Otolaryngol Col Soc Mexican Republic, 2008.
- Freche C, Castillo S, De Corbiere S et al (1998): Usefulness of hypertonic seawater (Sinomarin®) in rhinology; Rev Offic de la Societ Franc de ORL, 1998; 50: 73-75.
- Köksal T, Çizmeci MN, Bozkaya D, Kanburoğlu MK, Şahin Ş, Taş T, et al. Comparison between the use of saline and seawater for nasal obstruction in children under 2 years of age with acute upper respiratory infection. Turk J Med Sci, 2016; 46(4): 1004-1013. doi: 10.3906/sag-1507-18. PMID: 27513397.
- Furmaniak MA, Misztak AE, Franczuk MD, Wilmotte A, Waleron M, Waleron KF. Edible Cyanobacterial Genus Arthrospira: Actual State of the Art in Cultivation Methods, Genetics, and Application in Medicine. Front Microbiol, 2017; 8: 2541. doi: 10.3389/fmicb.2017.02541. PMID: 29326676; PMCID: PMC5741684.
- Fitton JH. Therapies from fucoidan; multifunctional marine polymers. Mar Drugs, 2011; 9(10): 1731-1760. doi: 10.3390/md9101731. Epub 2011 Sep 30. PMID: 22072995; PMCID: PMC3210604.
- Fitton JH, Stringer DS, Park AY, Karpiniec SN. Therapies from Fucoidan: New Developments. Mar Drugs, 2019; 17(10): pii: E571. doi: 10.3390/md17100571.
- Hans N, Malik A, Naik S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour Technol Rep, 2021; 13: 100623. doi: 10.1016/j.biteb.2020.100623. Epub 2020 Dec 29. PMID: 33521606; PMCID: PMC7836841.
- Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun, 1997; 65(1): 1-8. doi: 10.1128/iai.65.1.1-8.1997. PMID: 8975885; PMCID: PMC174549.
- Wang J, Jin W, Hou Y, Niu X, Zhang H, Zhang Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int J Biol Macromol, 2013; 57: 26-29. doi: 10.1016/j.ijbiomac.2013.03.001. Epub 2013 Mar 13. PMID: 23500437.
- Dhakad AK, Pandey VV, Beg S, Rawat JM, Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J Sci Food Agric, 2018; 98(3): 833-848. doi: 10.1002/jsfa.8600. Epub 2017 Sep 11. PMID: 28758221.
- Mahendran G, Verma SK, Rahman LU. The traditional uses, phytochemistry and pharmacology of spearmint (Mentha spicata L.): A review. J Ethnopharmacol, 2021; 278: 114266. doi: 10.1016/j.jep.2021.114266. Epub 2021 Jun 1. PMID: 34087400.
- Afonso AF, Pereira OR, Cardoso SM. Health-Promoting Effects of Thymus Phenolic-Rich Extracts: Antioxidant, Anti-Inflammatory and Antitumoral Properties. Antioxidants (Basel), 2020; 9(9): 814. doi: 10.3390/antiox9090814. PMID: 32882987; PMCID: PMC7555682.
- Gavliakova S, Biringerova Z, Buday T, Brozmanova M, Calkovsky V, Poliacek I, Plevkova J. Antitussive effects of nasal thymol challenges in healthy volunteers. Respir Physiol Neurobiol, 2013; 187(1): 104-107. doi: 10.1016/j.resp.2013.02.011. Epub 2013 Feb 21. PMID: 23438788.
- Lim M, Lew-Gor S, Darby Y, Brookes N, Scadding G, Lund VJ. The relationship between subjective assessment instruments in chronic rhinosinusitis, Rhinology, 2007; 45(2): 144-147. PMID: 17708462.

