Intracranial Malignant Myoepithelioma: A Rare Aggressive Tumor: Case Report and Literature Review
Nagarjun Ballari*
Department of Radiation Oncology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Received Date: 29/11/2021; Published Date: 27/12/2021
*Corresponding author: Nagarjun Ballari, Department of Radiation Oncology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Introduction
Myoepithelial tumors are very rare tumors and can be benign or malignant. Salivary glands are the classical organs of origin of myoepithelial tumors, although they are known to occur in in breast, skin, soft tissues as well as lungs. Central Nervous System (CNS) is an extremely rare site of presentation of these tumors and in the CNS, sellar region is the commonest location. Myoepitheliomas may arise from the remnants of the Rathke’s cleft. Around ten such cases of intracranial myoepithelioma have been reported in the literature. With the advent and increased availability of immunohistochemical markers, there has been an increase in the number of cases of myoepithelial tumors diagnosed in the last decade. Here we report the aggressive clinical course of an intracranial malignant myoepithelial tumor in a young male patient.
Case Report
A 28-year-old male presented with complaints of holo-cranial headache, which was insidious onset, gradually progressive, moderate to severe in intensity for a duration of 3 months, without any history of trauma, not relieved with over-the-counter medications. There was also associated history of nausea and vomiting, unrelated to food intake for a month. Subsequently, the patient developed altered sensorium for 2 weeks. There were no other neurological symptoms. There was a history of fatal malignancy in his father and cancer of head and neck in his sister. Exact diagnosis and treatment history was unavailable for both.
General physical examination revealed multiple café au lait spots noted over the back. There was no organomegaly or lymphadenopathy. Patient was alert, conscious, co-operative, not oriented to time, place and person and there were no signs of any focal neurological deficit. A Computed Tomography (CT) scan was suggestive of an ill-defined non encapsulated heterodynes lesion with patchy calcified foci within and moderate perilesional edema in left temporo-parietal parenchyma associated with midline shift. On contrast administration, heterogenous enhancement was noted. For confirmation of CT findings, a contrast enhanced magnetic resonance imaging (CEMRI) was done. The patient underwent temporo-parietal craniotomy followed by gross total excision. Intraoperatively, the tumor was hard, not sackable, and moderately vascular with calcification and showed an intertumoral cyst.
On histopathology, a biphasic tumor was noted with both epithelial and stromal components. The epithelial component was arranged in the form of glands, cribriform pattern and papillae. The cells were cuboidal to columnar, with high nucleo-cytoplasmic ratio, hyperchromatic nuclei, with frequent mitosis. The stromal component was composed of spindle to polygonal cells with coarse chromatin, distributed in a myxoid background with frequent mitosis. In addition to areas of necrosis and calcification, malignant chondroid and osteoid production was noted indicating chondrosarcomas and osteosarcomas differentiation. No other heterologous element was seen. On immunohistochemistry, the epithelial component stained positive for cytokeratin (CK), glial fibrillary acidic protein (GFAP), S100 and showed patchy p63 and calponin positivity indicating myoepithelial differentiation. S-100 and SATB2 stains were positive in chondrosarcomas and osteosarcomas components respectively. They were negative for desman and myozenin (excluding rhabdomyosarcoma), OLIG2 (excluding glial differentiation), OCT4, SALL4 and AFP (excluding germ cell tumor), synaptophysin and chromogranin (excluding neuroendocrine tumor), TLE and smooth muscle actin. Nuclear INI1 expression was retained. Fluorescence in situ hybridization (FISH) for SS18 gene rearrangement (using dual color break apart FISH probe, Vises) was negative, excluding synovial sarcoma. FISH for EWSR1 gene rearrangement (using dual color break apart FISH probe, vises) was carried out, the result was negative. The final pathological diagnosis was given as malignant mixed tumor (myoepithelioma) with osteosarcomas and chondrosarcomas elements (Figure 1, 2).

Figure 1: (A) Section shows a biphasic tumor with both epithelial and stromal components (Hematoxylin and Eosin, x100). (B) The epithelial component is in form of glands and papillae, lined by cuboidal to columnar cells (Hematoxylin and Eosin, x200). (C) The stromal cells are spindled to bizarre cells, in a myxoid stroma (Hematoxylin and Eosin, x100). (D) The stroma shows chondroid elements (Hematoxylin and Eosin, x100).
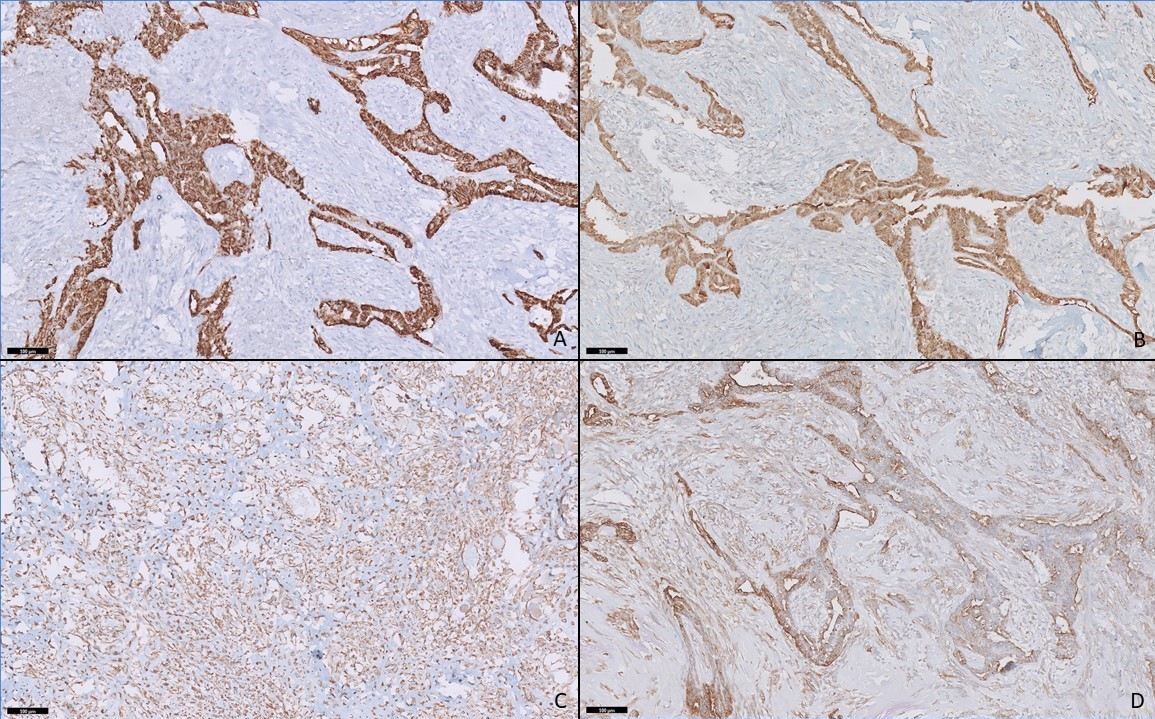
Figure 2: Panel of immunohistochemistry. The epithelial component shows positivity for cytokeratin (A) and S100 (B). The stromal component is positive for vimentin (C). Both epithelial and stromal components show positivity for smooth muscle actin (D) (Immunohistochemistry, x200).
Post-operatively, FDG PET-CT was suggestive of a localized heterogeneously enhancing residual soft tissue mass (3X3cm, SUVmax 15.4), in the left temporo-parietal lobe, adjoining the occipital horn of left lateral ventricle. There was no tracer uptake elsewhere in the body. MRI brain done at 6 weeks confirmed the similar findings. Screening spine and CSF analysis revealed no evidence of drop metastases. Blood and CSF analysis performed were essentially normal and showed no elevations of tumor markers.
The patient was planned for adjuvant radiation to a total dose of 54Gy at 1.8Gy per fraction. However, two weeks after initiation of radiotherapy, the patient developed multiple episodes of seizures which were managed conservatively. A CECT done at this point of time revealed a large progressive tumor in the site of residual disease. The patient had a protracted clinical course in which he deteriorated and succumbed to the disease within a week.
Discussion
Myoepithelial tumors are very heterogenous in terms of histology, cytological morphology and immunophenotype. Besides their original described origin in salivary glands, literature has shown them to originate in various sites like breast, skin, lacrimal gland, head and neck including paranasal sinus and vulva. Intracranial myoepitheliomas have been rarely reported in the literature. Despite extensive literature search, only ten cases have been found of which half were malignant (Table 1).
Table 1: Myoepithelial tumors of central nervous system.

Intracranial myoepitheliomas have been reported in patients from 1 year [6] to 52 years [5] of age, both in intra as well as extra-axial regions. Here, the radiological differential diagnosis in this index case was glioma considering the heterogenous enhancement with extensive perilesional edema.
The pathological appearance was also atypical. The stroma was heterogenous including chondroid and osseous metaplasia as has also been reported by Ghanta et al. [5]. In malignant cases, high mitotic index has been studied with mitosis as high as 11/10 high power field [3]. There are still date no specific criteria to define them as benign or malignant and it depends primarily on the clinical characteristics.
Myoepitheliomas and their malignant counterpart, myoepithelial carcinoma phenotypically resembles the myoepithelial cells. However, the neoplastic myoepithelial cells are morphologically heterogenous. Myoepitheliomas are lobulated tumors composed of cords of epithelioid or spindle cells with reticular architecture and chondromyxoid, collagenous and hyalanoid stroma. Variable epithelial component can be identified, as seen in the index case. Pure myoepitheliomas and mixed cell tumors lie on a morphological continuum with overlap in histological appearance and clinical behavior. The tumors, on immunohistochemistry, express a wide range of epithelial and mesenchymal markers, similar to myoepitheliomas of other primary sites. Cytokeratin and S100 are almost always positive as is seen in our case. Epithelial membrane antigen, glial fibrillary acidic protein and smooth muscle actin appear to be variably expressed.
Around 45% of soft tissue myoepithelial tumors show EWSR1 gene translocation but pleomorphic adenoma gene (PLAG-1) mutations are commonly observed in salivary gland myoepitheliomas [10,11]. In the available literature, genetic studies were done in only two intracranial cases to detect EWSR1 mutations, of which only one was positive [6]. In our case, EWSR1 gene mutation was done although it yielded a negative report.
The origin of intracranial myoepitheliomas is a matter of concern. In and around the sella, they are postulated to originate from the salivary gland rests in posterior pituitary which communicate with remnants of oropharynx in Rathke’s pouch [9,12]. Tumors in middle cranial fossa may arise from ectopic salivary gland tissue remnants in middle ear [13].
There are conflicting reports regarding the optimal strategy for myoepithelial neoplasm. For localized disease, wide local excision is the mainstay of treatment. Is there any information regarding the extent of surgery and survival? Radiation therapy has been reported as an adjunctive therapy following subtotal resection or in cases of recurrence; kindly add about radiation sensitivity, radiation doses used and techniques that are preferable. If there are no details available in the literature mention the same citing that this lack of information is one of the reasons these tumours show poor results. However, given the rarity, its efficacy has not been well defined. They may be characterized by nodal metastasis which may be addressed surgically. There is no proven role of chemotherapy in such tumors. Can u find any details regarding the various chemotherapeutic agents that have been used either as adjuvant or palliative? If no information is present, kindly add chemotherapy agents used for extra cranial myoepitheliomas and that if they can be used in CNS MYO tumors [9].
Conclusion
Intracranial myoepitheliomas are rare. Surgical approaches require careful planning to minimize morbidity. Although commonly benign and slow growing, myoepitheliomas may invade bone and dura and in rare instances differentiate into frankly malignant lesions with metastatic potential. A metastatic workup to look for secondaries is essential. Surgical excision is the mainstay of therapy, with radiation therapy reserved for unresectable or recurrent lesions. Chemotherapy has not been effective in the management of myoepithelial neoplasms, but is reserved for unresectable, progressive, or metastatic myoepithelial carcinomas. The present case scenario solidifies the fact that conventional approach via surgery and adjuvant radiotherapy is in adequate in achieving any kind of tumour control. Further research in terms of molecular pathogenesis, identification of molecular targets for therapy, radiobiological studies to identify the radiation sensitivity of these tumours may enable a more aggressive and tailored approach towards these rare tumours.
References
- Erdogan S, Rodriguez FJ, Scheithauer BW, Abell-Aleff PC, Rabin M. Malignant myoepithelioma of cranial dura. Am J Surg Pathol, 2007; 31: 807-811.
- Vajtai I, Hewer E, Neuenschwander M, Schäfer SC, Kappeler A, Lukes A. Myoepithelioma of the cerebellopontine angle: A previously not documented salivary gland type neoplasm within the cranium. Clin Neuropathol, 2013; 32; 176-182.
- Hong Y, Guo SX, Chen S, Klebe D, Zhang JM, Wu Q. Rapid- developed primary malignant myoepithelioma in the cavernous sinus: A case report. BMC Neurol, 2013; 13: 40.
- Hayward DM, Yoo D, Lee JM, Wild E, Prabhu VC. Myoepithelioma of the orbital apex and middle cranial fossa: Case report and review of the literature. Neuroophthalmol, 2014; 38: 14-20.
- Ghanta RK, Uppin MS, Koti K, Hui M, Uppin SG, Mukherjee KK. Primary intracranial Parachordoma: An unusual tumor in brain. Surg Neurol Int, 2014; 5: S506-S511.
- Choy B, Pytel P. Primary intracranial myoepithelial neoplasm: A potential mimic of meningioma. Int J Surg Pathol, 2016; 24: 243-247.
- Gupta K, Klimo P Jr, Wright KD. A 2-Year-old girl with dysmetria and ataxia. Brain Pathol, 2016; 26: 126-127.
- Gowripriya G, Sridhar K, Vij M. Intracranial Myoepithelioma: A Case Report and Review of Literature. Neurology India, 2019; 67(5): 1347.
- Nieder C, Schneller F, Grosu A, Peschel C, Molls M. Radiotherapy and Chemotherapy for Myoepithelioma of the Sellar Region. Strahlentherapie und Onkologie, 2005; 181(4): 260-263.
- Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer, 2010; 49: 1114-1124.
- Aström AK, Voz ML, Kas K, Röijer E, Wedell B, Mandahl N, et al. Conserved mechanism of PLAG1 activation in salivary gland tumours with and without chromosome 8q12 abnormalities: Identification of SII as a new fusion partner gene. Cancer Res, 1999; 59: 918-923.
- Chimelli L, Gadelha MR, Une K, Carlos S, Pereira PJ, Santos JL, et al. Intra-sellar salivary gland like pleomorphic adenoma arising within the wall of a Rathke's cleft cyst. Pituitary, 2000; 3: 257-261.
- Curry B, Taylor CW, Fisher AW. Salivary gland heterotopia: A unique cerebellopontine angle tumour. Arch Pathol Lab Med, 1982;106: 35-38.

