Aggressive Ocular Merkel Cell Carcinoma: A New Case Report
Mohamed Réda KHMAMOUCHE*, Mohamed Amine Essaoudi, Mohamed Allaoui, Rachid Tanz, Mehdi Toreis, Mohamed Fetohi, Nawfel Mellas and Mohamed Ichou
Medical oncology department, Military Hospital Mohammed V, Morocco.
Medical oncology department, Hassan II University Hospital, University Sidi Mohamed Ben Abdellah, Fez, Morocco.
Pathology department, Military Hospital Mohammed V, Rabat, Morocco.
Medical oncology department, Military Hospital Moulay Ismail, University Sidi Mohamed Ben Abdellah Fez, Morocco.
Received Date: 28/11/2021; Published Date: 20/12/2021
*Corresponding author: Dr. Mohamed Réda KHMAMOUCHE, Medical oncologist, Medical oncology department, Military Hospital MOHAMED V, Rabat, University SIDI MOHAMED BEN ABDELLAH, Fez, Morocco.
Abstract
An 80-year-old woman without past medical or surgical history was presented in with a huge mass taking the whole left eye. An excisional biopsy was performed. Histopathology showed Merkel Cell Carcinoma, which is a rare and aggressive malignant cutaneous tumor. It can affect the eyelids and is simulate benign lesions which can delay establishment of suitable therapy. A correct histopathological analysis, including immunohistochemistry techniques and oriented by clinical suspicion, is important for the correct diagnosis. Prognosis of this tumor is known to be poor, that is the reason why an accurate diagnosis and an early multidisciplinary team with oncologists, ophthalmologists and physician radiation are essential to a successful management of this rare disease.
Keywords: Merkel cell carcinoma; Eyelid; Immunochemistry; Chemotherapy; Radiotherapy; Prognosis
Abbreviations: MCC: Merkel Cell Carcinoma; HIV: Human Immunodeficience Virus; MRI: Magnetic Resonance Imaging; CK20: Cytokeratin 20; EMA: Epithelial Membran Antigen; CK CAM: Cytokeratin Cell Adhesion Molecule; TTF-1: Thyroid Transcription Factor-1; CK20: Cytokeratin 20; LCA: Leukocyte Common Antigen; MRI: Magnetic Resonance Imaging; CPIs: Checkpoint Inhibitors
Introduction
Merkel Cell Carcinoma [MCC] is a rare and aggressive malignant tumor of the skin. It was first described in 1972 by Taker in 1972, referring to it as ‘’trabecular carcinoma of the skin.” Merkel cells are dermal mechanoreceptors that have been described as capable of malignant transformation and thought to be the origin of MCC [1]. Established risk factors for MCC include advanced age > 65 years old, immunosuppression and significant sunlight exposure, and the infection of Merkel cell polyomavirus was recently identified in 80% of cases of MCCs [2-4]. In particular, there is an increased relative risk of developing MCC in people with concurrent HIV infection [5].
Recently, there has been an increase of incidence of this rare disease [6]. Diagnosis most often occurs after 60 years-old, with age specific rates highest over the age of 70 years [7-9] with a slight male predominance [7-11]. About one tenth of Merkel cell carcinoma cases occur in the eyelid and periocular regions [12], usually affecting elderly people [13]. These lesions localizations of MCC have an aggressive natural history and potential for local recurrence, lymphatic node metastasis, and spread metastasis dissemination. [13]
MCC lesions typically appear as asymptomatic, solitary nodules with distinctive red, pink or violaceous coloring and may have overlying ulceration or telangiectasia [14]. The diagnosis of this tumor must be histopathological by biopsy because its rarity and the possibility of simulating benign lesions wish can delay in treatment [12].
These neoplasms have a poor prognosis. The estimated mortality rate for all patients with MCC is less than 35% [15].
Reports of ophthalmological involvement include principally primary tumors of the eyelid, but also metastases to the orbit, ciliary, choroid, body, iris and conjunctiva have been described [16]. Treatment approaches of ophtalmological MCC are difficult and require a skilled and experienced multidisciplinary team [17].
Case Report
An 80-year-old female without past medical or surgical history was presented in our institution with a huge mass taking the whole left eye. The mass was reddish, vascularized and painless, developed from the upper eyelid of the left eye 3 months ago [Figure 1]. According to the patient and her family, the lesion was growing rapidly. MRI [Magnetic resonance imaging] showed no invasion in orbit, but the results were compatible with a malignant eyelid tumor. She underwent incisional biopsy of the mass by local anesthesia of the mass. Histopathology showed features of a high-grade non-differentiated carcinoma with a trabecular aspect [Figure 2], Immunohistochemistry revealed CK 20 and chromogranin positive [Figure 3]. In addition, EMA expression and CD 56 or cytokeratin CAM [ cell adhesion molecule] were positive by immunopathology [Figure 4]. There was no immunoreactivity to protein S-100, thyroid transcription factor 1[TTF-1] and leukocyte common antigen [LCA]. Thus, the tumor was diagnosed as a MCC. The patient’s condition deteriorated rapidly and she required palliative care. The patient died 1 month after diagnosis.
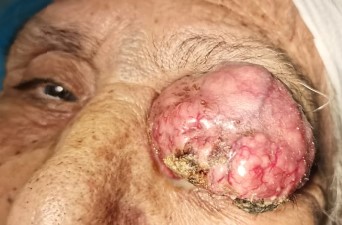
Figure 1: external photograph in a patient showing a red and vascularized mass of the upper eyelid taking the whole left eye: ocular Merkel cell carcinoma.

Figure 2: Histopathology [hematoxylin and eosin] HEx25: Histopathology showed features of a high-grade non-differentiated carcinoma with a trabecular aspect.
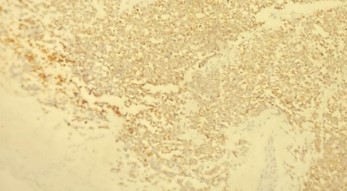

Figure 3: Photomicrograph showing the same tumor stained for CK20. [A] There is strong expression of CK20 in the cytoplasm and membrane of MCC. [Gx25] and [B]: strong positivity of chromogranin [Gx40].


Figure 4: By immunohistopathology, [A] the tumor displays CD 56 or CAM [Gx25] and [B] EMA [Epithelial membrane antigen] positive cells [Gx10] in Merkel cell carcinoma.
Discussion
Merkel cell carcinoma is a frequently lethal cutaneous cancer that has a high propensity for lymphatic metastases and local recurrence, has poor prognosis. Several reports have described the association of MCC of the eyelids [17] with incidence between 5% and 20% of all cases of head and neck Merkel cell carcinoma [8,10,13]. Most commonly in the eyelid, the tumor affects the upper eyelid and develops near the eyelid margin, sometimes causing partial or complete eyelash loss. The skin covering the primary lesion is habitually hard, smooth-surfaced, and red, with hues ranging from pink to violaceous. Overlying telangiectasis is often present [13,19,20].
Clinical diagnosis of these tumors is difficult because they can present as benign lesions such as chalazion and keratoacanthoma.[12] or malignant tumors as basal cell carcinoma [9,21].
MCCs of eyelids tumors are usually treated initially as benign lesions, which leads to local recurrences. In addition, in many cases the histopathologic examination is not performed, resulting in a delay of the exact diagnosis.[12,19].
Merkel cell carcinoma requires aggressive and rapid initial therapy for a favorable outcome. Owing to the rarity of this tumor, it is difficult to conduct a randomized, prospective
study to define optimal treatment [22]. The standard of care for primary tumor is surgical resection with negative margins or Mohs micrographic surgery with or without adjuvant radiotherapy [14,15,22]. A wide excision with margins of 2,5 to 3 cm has been advocated and is based on studies showing a significant reduction in local recurrence rate by increasing the margins from 1 to 3 cm [12,22].
The role of radiotherapy in the management of eyelid MCC remains controversial. There is a decrease of local recurrence and improving survival rate after radiotherapy [23,24]. However, this has no effect on overall survival [25,26]. Currently, most eyelid MCCs are treated without irradiation. On the contrary, adjuvant chemotherapy for MCC has not shown benefits and has even been associated with increased morbidity and mortality. Even though patients may initially respond, resistance often develops [27]. Chemotherapy in MCCs is used in patients with metastasis or locally advanced tumors. Moreover, a high incidence of toxic death occurs due to chemotherapy. [28].
MCC has demonstrated sensitivity to chemotherapeutic drugs such as cisplatin, cyclophosphamide, doxorubicin, vincristine, etoposide and 5-fluorouracil [22,28]. A retrospective review of 107 patients with locally advanced or metastatic MCC demonstrated overall clinical response rate to first-line chemotherapy of 61%, however, this study additionally demonstrated high toxicity-related mortality [28]. Combination chemotherapy is more effective than monochemotherapy [28].
Cisplatin plus etoposide, cyclophosphamide plus doxorubicin plus vincristine, or cyclophosphamide plus epirubicin plus vincristine are the most commonly used regimens [17]. The response rate is 70%, with a complete response in 35%. Interestingly, non-ocular MCC is reported to be a very aggressive tumor, lethal in 33% of patients.[17] Myelosuppression, sepsis, fatigue, alopecia, diarrhea, nausea and renal failure are the most common adverse events of chemotherapy reported in literature [29].
The development of new alternative therapies, such as immunotherapy with checkpoint inhibitors [CPIs], has shown good results in the treatment for advanced or metastatic diseases. and, consequently, CPIs are considered to be emerging immunotherapeutic options for these patients [29].
MCC of the head and neck has been shown to be particularly aggressive with higher likelihood of cartilage, bone, and skeletal muscle invasion when compared to MCC of other sites [10]. While eyelid and periocular lesions may arise earlier due to better visualization of the lesion in this location, MCC of the head and neck still demonstrates high incidence of spread to the regional lymph nodes with up to 2/3 of patients having regional lymph node metastasis and 1/3 of patients with distant metastasis within 18 months of diagnosis [13,14,21]. MCC of the eyelid has been reported to have an overall metastatic rate ranging from 10% to 30% [14,21,30], regional lymph node recurrence rate of 20%, and distant metastasis rate of 5%. The recent study by our group demonstrated a 22% metastatic rate overall which included an 11% nodal metastasis and 11% distant metastasis rate [14].
Distant metastasis from MCC regardless of anatomic sites most frequently occurs in the skin, bone, brain, liver, and lung [8,10,13]. The reported rate of distant metastasis is as high as 36% [21].
Conclusion
Merkel cell carcinoma of the eyelid is associated with significant rates of recurrence and metastases, as well as a significant mortality rate. This localization appears to be associated with a better prognosis, which may be related to earlier detection.
Surgery followed by radiation therapy is considered to be the first-line treatment for primary or loco-regional MCC in order to prevent recurrences and lymph node metastasis, while chemotherapy has always been used to treat advanced and metastatic forms.
Consent
Informed consent was obtained from the patient for publication of this case report and accompanying images.
Conflicts of interest
The authors declare are no conflicts of interest.
References
- Toker C. Trabecular carcinoma of the skin. Arch Dermatol, 1972; 105: 107-110.
- Becker JC. Merkel cell carcinoma. Ann Oncol, 2010; 21[suppl 7]: vii81-vii85.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol, 2003; 49[5]: 832-841.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg, 2013; 39[2]: 232-238.
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet 2002; 359: 497-498.
- Lemos B, Nghiem P. Merkel cell carcinoma: More deaths but still no pathway to blame. J Invest Dermatol, 2007; 127: 2100-2103.
- Hodgson NC. Merkel cell carcinoma: Changing incidence trends. J. Surg. Oncol, 2005; 89: 1–4.
- Lemos BD, Storer BE; Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J. Am. Acad. Dermatol, 2010; 63: 751–761.
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol, 2008; 58: 375–381.
- Smith VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: Identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope, 2012; 122: 1283–1290.
- Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Allen PJ, et al. Five hundred patients with merkel cell carcinoma evaluated at a single institution. Ann. Surg, 2011: 254: 465–475.
- André GBN, Suzana M, Ruth MS, Veralucia R. Eyelid Merkel Cell Carcinoma: Report of Three Cases. Ophthalmic Plastic and Reconstructive Surgery; 2004; 20(2): pp 117–121.
- Kivela T, Tarkkanen A. The Merkel cell and associated neoplasms in the eyelids and periocular region. Surv Ophthalmol, 1990; 35: 171–187.
- Helen M., Matthew C. S. and Bita E. Merkel Cell Carcinoma of the Eyelid and Periocular Region. Cancers, 2014 ; 6 : 1128- 1137. DOI:10.3390/cancers6021128
- Hitchcock CL, Bland KI, Laney RG III, Franzini D, Harris B, Copeland EM III. Neuroendocrine [Merkel cell] carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg.1988;207[2]:201-207
- Kirwan C, Carney D, O’Keefe M. Merkel cell carcinoma metastasis to the iris in a 23-year-old female. Ir Med J, 2009; 102: 53-54.
- Luxia Chen, Limin Zhu, Jianguo Wu, Tingting Lin, Baocun Sun, Yanjin He. Giant Merkel cell carcinoma of the eyelid: a case report and review of the literature. World Journal of Surgical Oncology, 2011 ; 9: 58.
- Satoru K, KAN I, et al. Merkel Cell Carcinoma of the Conjunctiva. Ophthalmology, 2010; 117.
- Rubsamen PE, Tanenbaum M, Grove AS, et al. Merkel cell carcinoma of the eyelid and periocular tissues. Am J Ophthalmol, 1992; 113: 674–680.
- Metz AK, Jacob M, Schmidt U, et al. Merkel cell carcinoma of the eyelid: histological and immunohistochemical features with special respect to differential diagnosis. Graefs Arch Clin Exp Ophtalmol, 1998; 236: 561–566.
- Peters GB, Meyer DR, Shields JA, Custer PL, Rubin PA, Wojno TH, et al. Management and prognosis of merkel cell carcinoma of the eyelid. Ophthalmology, 2001; 108: 1575–1579.
- Ott MJ, Tanabe KK, Gadd MA, et al. Multimodality management of Merkel cell carcinoma. Arch Surg 1999; 134: 388–393.
- Plunkett TA, Subrumanian R, Leslie MD, Harper PG: Management of Merkel cell carcinoma. Expert Rev Anticancer Ther, 2001; 1: 441-445.
- Meeuwissen JA, Bourne RG, Kearsley JH: The importance of postoperative radiation therapy in the treatment of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys, 1995; 31: 325-331.
- Poulsen MG, Rischin D, Porter I, Walpole E, Harvey J, Hamilton C, Keller J, Tripcony L: Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys, 2006; 64: 114-119.
- Francisco L, Federico Andrés C, Eugenia P, Pablo C. Merkel cell carcinoma of the cornea and conjunctiva. Latin American Journal of Ophthalmology, 2020; 3(7).
- Garneski KM, Nghiem P. Merkel cell carcinoma adjuvant therapy: Current data support radiation but not chemotherapy. J Am Acad Dermatol, 2007; 57: 166-169.
- Voog E, Biron P, Martin JP, Blay JY: Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer 1999; 85: 2589-2595.
- Villani A, Fabbrocini G, Costa C, Annunziata MC, Scalvenzi M. Merkel cell carcinoma: Therapeutic update and emerging therapies. Dermatol Ther [Heidelb], 2019; 9: 209-222.
- Missotten GS, de Wolff-Rouendaal D, de Keizer RJ. Merkel cell carcinoma of the eyelid review of the literature and report of patients with merkel cell carcinoma showing spontaneous regression. Ophthalmology 2008; 115: 195–201.

