Leiomyosarcoma of Adrenal Gland: A New Case Report with Literature Review
Mohamed Réda KHMAMOUCHE, Mohamed Amine Essaoudi, Tarik Mahfoud, Rachid Tanz, Aziz Bazine, Mohamed Fetohi, Nawfel Mellas and Mohamed Ichou
Medical oncology department, Military Hospital Mohammed V, Rabat, Morocco.
Medical oncology department, Hassan II University Hospital, University Sidi Mohamed Ben Abdellah, Fez, Morocco.
Pathology department, Military Hospital Mohammed V, Rabat, Morocco.
Medical oncology department, Military Hospital Moulay Ismail, Meknes, Morocco.
Received Date: 28/11/2021; Published Date: 20/12/2021
*Corresponding author: Mohamed Réda KHMAMOUCHE, Medical oncologist, Medical oncology department, Military Hospital MOHAMED V, Rabat, Morocco.
Introduction
Primary Adrenal Leiomyosarcoma (PAL) is a very rare soft tissue neoplasm that derives from the smooth muscle [1,2]. This mesenchymal malignant tumor has been described rarely in the literature and is without any specific findings by tests of laboratory or radiologic imaging modalities [3]. Its diagnosis is based completely on the results of both histological and immunohistochemical evaluations, which are essential not only for determining the tumor type but also for predicting the biological behavior [4].
Primary adrenal leiomyosarcoma is a high-grade proliferating tumor with a high potentiel risk of metastasis. PAL have no specific clinical symptoms and does not express or secretes adrenal hormones and no characteristic tumor biomarkers have been identified [5]. Therefore, the preoperative diagnosis of PAL is difficult, and the tumor is often discovered at an advanced stage [5].
The first patient with PAL was reported by Choi and Liu in 1981 [6]. Herein, we present a rare case of primary adrenal leiomyosarcoma in a 60-year-old woman with incidentally discovered PAL after surgery, along with review of the current knowledge of the clinical, radiological, and histopathological characteristics of this malignant tumor.
Case Report
A 60 -year-old woman with no previous medical or surgical history, presented with severe intermittent abdominal pain essentially on the upper left abdominal quadrant. She denied any other symptoms, as well as recent weight loss, fever, nausea or vomiting.
Family history was non-contributory. The initial physical examination showed no abnormal findings, except for mild abdominal discomfort at palpation of left hypocondrium. No mass was palped. She had a pulse of 72/minute and blood pressure of 130/70 mm Hg. There was no evidence of pedal edema or lymphadenopathy. A computed tomography was performed, which revealed a well-circumscribed heterogeneously mass measuring 9×8×8 cm located in the left suprarenal areal.
Routine laboratory testing revealed no abnormal findings. The 24 hours urinary collections for cortisol and catecholamines were normal, as were serum aldosterone and ACTH levels. Based on a clinical diagnosis of non-functional adrenal tumor, a left open radical adrenalectomy adrenalectomy was conducted by the urologist surgeon. The postoperative period was uneventful and the patient was discharged on day 4 after surgery.
Gross pathological examination showed a roundish, white mass measuring 9×7 cm. The tumor was encapsulated with a firm consistency closely adherent to the adrenal gland. There were areas of hemorrhage without necrosis. The microscopic examination revealed a hypercellular tumor with intersecting fascicled of spindled cells (Figure 1). Resection margins were free of disease.
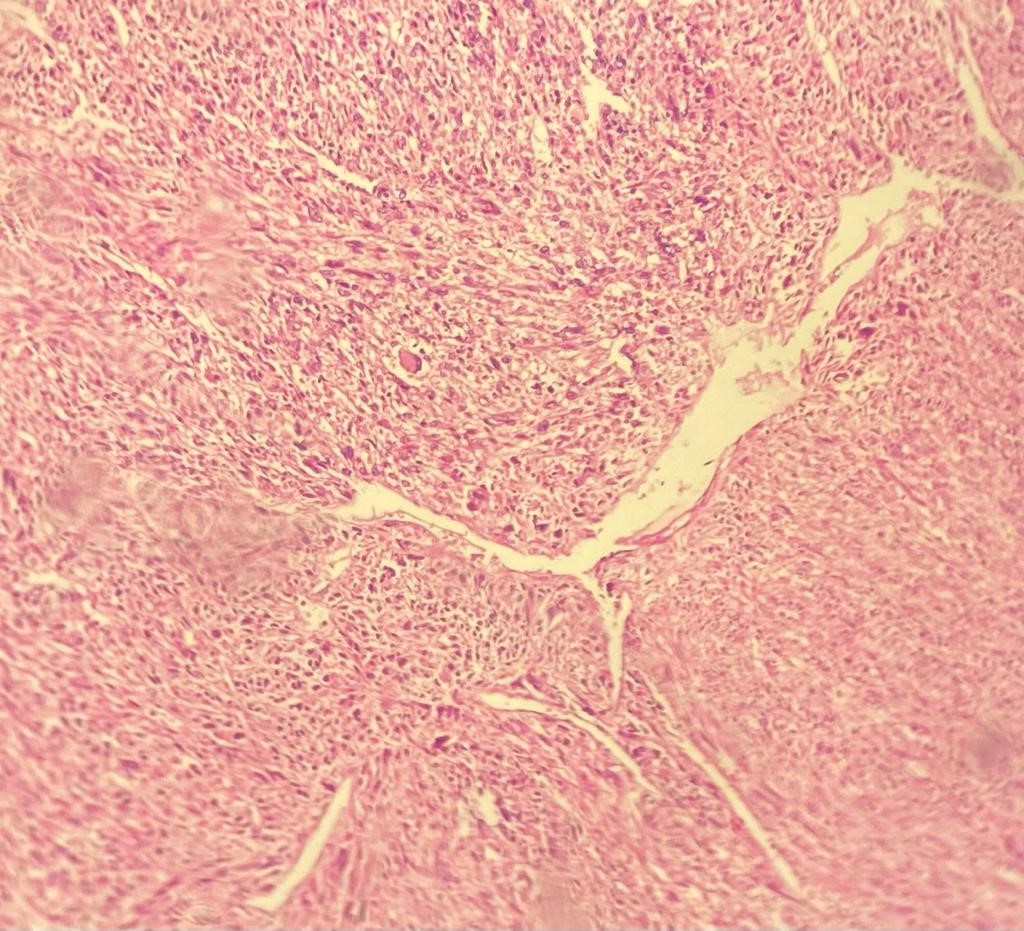
Figure 1: Microscopic details of tumor hypercellular tumor with intersecting fascicled of spindled cells (Hemalun Eosin, ×25).
On immunohistochemical studies, the tumor cells stained strongly positive for H- Caldesmon (Figure 2). Desmin and Smooth Muscle Actin (SMA) were also positive. The proliferation rate ki67 was high:60%. Based on the histopathological and immunohistochemical findings, the diagnosis of grade III primary adrenal leiomyosarcoma grade III was made.
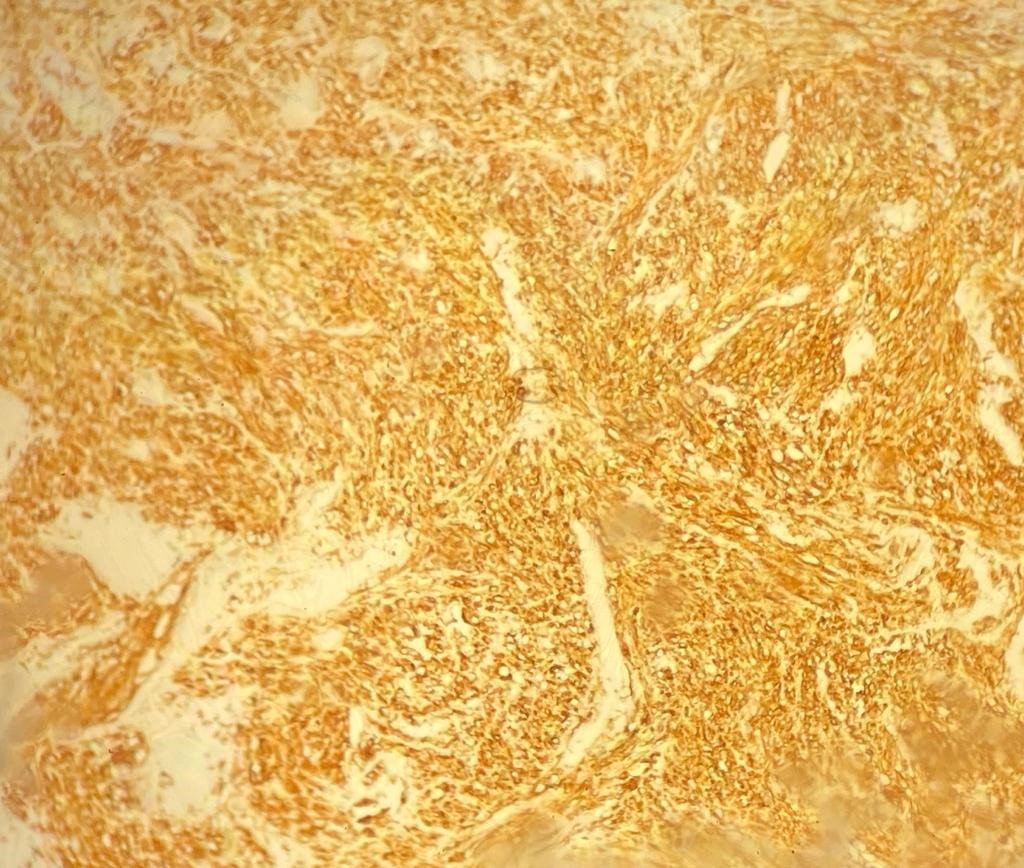
Figure 2: Strong positivity H-Caldesmon immunostaining (×10).
Then, the patient was therefore referred to our medical oncology department for adjuvant treatment one month after surgery. The patient was OMS at 1, SC was 1,8 m2. Subsequent CT of the chest and abdomen revealed a gross liver metastasis in segment VI measuring 5x4 cm with multiple lung metastases (Figure 3). Liver function tests, renal function, blood test, serum amylase and tumor markers ACE and CA-19.9 were also within normal limits. The cardiac exploratory was normal with 66% of left ventricular ejection fraction.
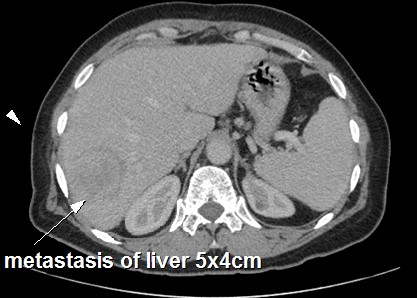
Figure 3: Computer tomography demonstrating liver metastasis of primary left adrenal leimyosarcoma after surgery.
After a multidisciplinary team, monochemotherapy with doxorubicin 75 mg/m2 was initiated. A total dose of 140 mg each 21 days was administred. Progression disease was objective after 6 cycles of treatment (Figure 4), and second line treatment by intravenous gemcitabine at dose 1000 mg/m2 over 30 minutes on days 1 and 8 every 21 days was initiated. The patient exhibited progression after 6 cycles by radiological evidence of liver and pulmonary metastases growing in number and in size (chest and abdomen CT) and the patient was placed to third line by docetaxel at dose of 100 mg/m2 and the patient is alive with metastatic disease 14 months after surgery.

Figure 4: Image CT scan showing progression disease with liver metastases after 6 cycles of the first line of palliative chemotherapy.
Discussion
Primary adrenal leiomyosarcomas are non‑functional mesenchymal malignant tumors of the adrenal gland, and are thought to arise from the smooth muscle wall of the central adrenal vein or its branches [2].
These tumors are very rare and represent only 0.1% to 0.2% of all retroperitoneal soft
tissue sarcomas in adults [7]. To the best of our knowledge, only approximately 45 cases have been reported in the English literature [5].
PAL can be asymptomatic and discovered incidentally during imaging performed for reasons unrelated to the PAL itself. The most common symptom at presentation is abdominal [5, 8-17] as was the case of our patient or flank pain [2,5,7,18,19]. Other symptoms include weight loss [17, 20-24] and lower extremity edema due to tumor growth into the inferior vena cava [5, 25].
They are typically found in image studies as large heterogeneous masses with no specific characteristics, which makes them undistinguishable from other adrenal tumors [10]. The etiology of adrenal leiomyosarcoma remains unknown, but HIV and EBV infections are suggested to be factors related to its occurrence [7].
There are no distinct biochemical tests that are useful to identify and confirm PAL preoperatively.
PAL do not produce any adrenal hormonal derangement and dosing adrenal hormones are consistently normal, and no specific tumor markers have been identified. Elevated concentration of neuronspecific enolase (NSE), which normalized postoperatively, was noted in one patient with PAL [26]. The level of serum NSE was significantly high preoperatively and NSE protein was highly expressed in the resected tumor. After surgery, serum NSE level became normal. It is suggested that serum NSE level could be a useful biomarker for the early detection for PAL.
Currently, the diagnosis of PAL is based on histopathological and immunohistochemical findings, showing a neoplasm consisting of spindle cells that stain positively for one or more of the following smooth muscle cell markers; SMA , desmin and vimentin [5] In addition to SMA and desmin, the tumor of our patient demonstrated positive immunoreactivity for H-caldesmon, also a marker of smooth muscle cells, in accordance with previous reports in patients with leiomyosarcomas of other origins [27]. The proliferation index Ki-67 was high (≥ 20 %) in our patient, which is in agreement with reported cases showing an index between 20% and 75%. [1,8,14,15,28]
The only and the best treatment modality of PAL is surgical resection with negative margins, after the evaluation of the tumor secretion to exclude the diagnosis of a functional tumor [29].
The histologic grade has been correlated with its biological behavior and prognosis (3), and these tumors rarely metastasize to regional lymph nodes, with metastases most frequently occured in the liver and lungs as our case report. Several cases have been reported where the tumor has invaded the inferior vena cava and caused a thrombosis [10, 25,30]
Postoperative adjuvant radiotherapy is suggested for locally advanced malignancies of soft tissue sarcomas [31]. However, the benefit of chemotherapy is very limited and can be used in cases of inoperable tumors, incomplete resections, and metastatic disease.
In metastatic disease, the longest survival of patients reported is three years after surgery [29].
Few single-agent chemotherapies have shown a reasonable response rate with acceptable toxicity to justify their use in the treatment of metastatic STS. Two of the most commonly used single agents are doxorubicin and ifosfamide [32].
Considering polychemotherapy, gemcitabine plus docetaxel can be considered in patients who cannot tolerate doxorubicin because of their cardiac history. The authors of the phase III study concluded that doxorubicin should remain the standard first-line treatment of metastatic soft tissue sarcomas, but gemcitabine/docetaxel can be considered an acceptable alternative in patients with cardiac dysfunction in whom doxorubicin is contraindicated. [33]
Trabectedin (Yondelis) is antineoplastic agent recently approved in the United States to treat advanced liposarcoma and leiomyosarcoma in patients who have already been treated with an anthracycline-based regimen.40 Trabectedin is a marine-derived tetrahydroisoquinolone alkaloid that binds to the minor groove of DNA, which disrupts the function of DNA binding proteins and leads to cell-cycle perturbation and apoptosis [34]. It is currently approved for administration via a central venous line at a dose of 1,5 mg/m2 IV over 24 hours on day 1 of a 21-day cycle. A multicenter, randomized, open-label, phase III trial compared trabectedin with dacarbazine in patients with advanced leimoyosarcoma or liposarcoma who had been treated previously with standard chemotherapy, including previous anthracycline-based chemotherapy [35]. Patients were randomized to receive either trabectedin 1,5 mg/m2 IV over 24 hours or dacarbazine 1000 mg/m2. The two drugs were administered on day 1 of a 21-day cycle. The primary endpoint was OS, with PFS as a secondary endpoint. Results of the trial showed significantly higher median PFS (4,2 months versus 1,5 months) with trabectedin compared with dacarbazine, without difference of median OS.
Pazopanib (Votrient) is a multitargeted tyrosine kinase inhibitor that targets and inhibits vascular endothelial growth factor receptors (VEGFR), PDGFR, fibroblast growth factor receptors, and c-Kit. It is indicated for the treatment of patients with advanced STS who have received prior chemotherapy, except for patients with liposarcoma [36] A randomized, double-blind, phase III study by the European Organization for Research and Treatment of Cancer compared pazopanib 800 mg orally daily until disease progression or intolerable toxicity with placebo in patients with various histological STS subtypes, patients with liposarcoma were excluded [37]. The primary endpoint was PFS, with OS as a secondary endpoint. Patients receiving pazopanib exhibited significantly better median PFS compared with placebo (4.6 months versus 1.6 months). However, no significant difference in OS was observed.
Regorafenib (Stivarga) is another multitargeted TKI that exerts its antineoplastic activity by targeting and inhibiting VEGFR, RET, c-Kit, BRAF, and PDGFR [38]. While regorafenib is not yet approved by the FDA for the treatment of non-GIST STS, its safety and efficacy have been evaluated in a randomized, placebo-controlled, phase II trial in patients with leimyosarcoma, liposarcoma, synovial sarcoma, or other types of STS that had been treated previously with a variety of agents [39]. Patients were randomized to receive regorafenib 160 mg orally daily for 21 days followed by seven days off or matching placebo. A total of 181 patients were randomized to treatment. The primary endpoint was PFS and OS was a secondary endpoint. Compared with placebo, regorafenib demonstrated a significant benefit in PFS among patients with leiomyosarcoma (3.7 months versus 1.8 months) and synovialo sarcoma (5.6 months versus 1.0 months). Patients with liposarcoma did not experience a benefit in PFS compared with placebo (1.1 months versus 1.7 months).
Conclusion
PAL is an extremely rare highly malignant tumor that is often diagnosed at an advanced stage.
The diagnosis is based on a combination of clinical, biological, radiological and immunohistochemical evidence. The gold standard of treatment is a complete surgical excision, with chemotherapy and radiation therapy being reserved for advanced disease.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this case report.
Ethics approval and consent to participate:
Oral patient consent was obtained for participation in this study.
Patient consent for publication:
Informed consent was obtained for the publication of patient data.
References
- Zhou Y, Tang Y, Tang J, Deng F, Gong G, Dai Y. Primary adrenal leiomyosarcoma: a case report and review of literature. Int J Clin Exp Pathol. 2015; 8(4): 4258-4263.
- Lack EE, Graham CW, Azumi N, et al. Primary leiomyosarcoma of adrenal gland. Case report with immunohistochemical and ultrastructural study. Am J Surg Pathol. 1991; 15(9): 899-905.
- Murat Tolga G, Asif Y, Berrin G, Ramazan G, Atis C, Cenk G, Turhan C. Primary Leiomyosarcoma of the Adrenal Gland: A Case Report with Immunohistochemical Study and Literature Review. Case reports in urology, 2014.
- Tomoya O, Yutaka Y, Tadahiko K, Noriyoshi M, Terutaka N, Toshio K, et al. Primary adrenal leiomyosarcoma with lymph node metastasis: a case report. World Journal of Surgical Oncology, 2016; 14: 176. DOI 10.1186/s12957-016-0936-z.
- Fatema J, Henri P, Lina H, Andreas M, Oskar R. Primary Adrenal Leiomyosarcoma: Clinical,Radiological, and Histopathological Characteristics. Journal of the endocrine society, 2020; 4(6): 1-9. DOI: 10.1210/jendso/bvaa055.
- Choi SH, Liu K. Leiomyosarcoma of the adrenal gland and its angiographic features: a case report. J.Surg Oncol. 1981; 16(2): 145-148.
- Nagaraj V, Mustafa M, Amin E, Ali W, Naji Sarsam S, Darwish A. Primary adrenal leiomyosarcoma in an arab male: a rare case report with immunohistochemistry study. Case Rep Surg. 2015; 2015: 702541.
- Mencoboni M, Bergaglio M, Truini M, Varaldo M. Primary adrenal leiomyosarcoma: a case report and literature review. Clin Med Oncol. 2008; 2: 353-356.
- Hamada S, Ito K, Tobe M, et al. Bilateral adrenal leiomyosarcoma treated with multiple local therapies. Int J Clin Oncol. 2009; 14(4): 356-360.
- Karaosmanoglu AD, Gee MS. Sonographic findings of an adrenal leiomyosarcoma. J Ultrasound Med. 2010;29(9):1369-1373.
- Kanthan R, Senger JL, Kanthan S. Three uncommon adrenal incidentalomas: a 13-year surgical pathology review. World J Surg Oncol. 2012; 10: 64.
- Deshmukh SD, Babanagare SV, Anand M, Pande DP, Yavalkar P. Primary adrenal leiomyosarcoma: a case report with immunohistochemical study and review of literature. J Cancer Res Ther, 2013; 9(1): 114-116.
- Bhalla A, Sandhu F, Sieber S. Primary adrenal leiomyosarcoma: a case report and review of the literature.Conn Med, 2014; 78(7): 403-407.
- Quildrian S, Califano I, Carrizo F, Daffinoti A, Calónico N. Primary adrenal leiomyosarcoma treated by laparoscopic adrenalectomy. Endocrinol Nutr, 2015; 62(9): 472-473.
- Onishi T, Yanagihara Y, Kikugawa T, et al. Primary adrenal leiomyosarcoma with lymph node metastasis:a case report. World J Surg Oncol, 2016; 14(1): 176.
- Taniguchi A, Ujike T, Fujita K, et al. [A Case of Adrenal Leiomyosarcoma]. Hinyokika Kiyo, 2017; 63(11): 465-469.
- Mulani SR, Stoner P, Schlachterman A, Ghayee HK, Lu L, Gupte A. First reported case of endoscopic ultrasound-guided core biopsy yielding diagnosis of primary adrenal leiomyosarcoma. Case Rep Gastrointest Med, 2018; 2018: 8196051.
- Alam MM, Naser MF, Islam MF, Rahman MA. Primary adrenal leiomyosarcoma in an adult female.Mymensingh Med J. 2014; 23(2): 380-383.
- Nerli RB, Ghagane S, Dixit NS, Hiremath MB, Deole S. Adrenal leiomyosarcoma in a young adult male. Int Cancer Conf J. 2020; 9(1): 14-17.
- Thamboo TP, Liew LC, Raju GC. Adrenal leiomyosarcoma: a case report and literature review. Pathology, 2003; 35(1): 47-49.
- Candanedo-Gonzalez FA, Vela Chavez T, Cerbulo-Vazquez A. Pleomorphic leiomyosarcoma of the adrenal gland with osteoclast-like giant cells. Endocr Pathol, 2005; 16(1): 75-81.
- Tomasich FD, Luz Mde A, Kato M, et al. [Primary adrenal leiomyosarcoma]. Arq Bras Endocrinol Metabol. 2008; 52(9): 1510-1514.
- Van Laarhoven HW, Vinken M, Mus R, Flucke U, Oyen WJ, Van der Graaf WT. The diagnostic hurdle of an elderly male with bone pain: how 18F-FDG-PET led to diagnosis of a leiomyosarcoma of the adrenal gland. Anticancer Res. 2009; 29(2): 469-472.
- Lee S, Tanawit GD, Lopez RA, Zamuco JT, Cheng BG, Siozon MV. Primary leiomyosarcoma of adrenal gland with tissue eosinophilic infiltration. Korean J Pathol. 2014; 48(6): 423-425.
- Etten B, van Ijken MG, Mooi WJ, Oudkerk M, van Geel AN. Primary leiomyosarcoma of the adrenal gland. Sarcoma. 2001; 5(2): 95-99.
- Goto J, Otsuka F, Kodera R, et al. A rare tumor in the adrenal region: neuron-specific enolase (NSE)-producing leiomyosarcoma in an elderly hypertensive patient. Endocr J, 2008; 55(1): 175-181.
- Hisaoka M, Wei-Qi S, Jian W, Morio T, Hashimoto H. Specific but variable expression of h-caldesmon in leiomyosarcomas: an immunohistochemical reassessment of a novel myogenic marker. Appl Immunohistochem Mol Morphol. 2001; 9(4): 302-308.
- Sakellariou M, Dellaportas D, Grapsa E, et al. Primary adrenal leiomyosarcoma: a case report. Mol Clin Oncol, 2020; 12(4): 317-320.
- Maria S, Dionysios D, Eirini G, Menelaos T, Athanasios D, Theodosios T, et al. Primary adrenal leiomyosarcoma:a case report. MOLECULAR AND CLINICAL ONCOLOGY, 2020; 12: 317-320. DOI: 10.3892/mco.2020.1987
- Doppalapudi SK, Shah T, Fitzhugh VA, Bargman V. Primary adrenal leiomyosarcoma with inferior vena cava extension in a 70-year-old man. BMJ Case Rep, 2019; 12(3). DOI: 10.1136/bcr-2018–227670
- Strander H, Turesson I, Cavallin-St˚ahl E. “A systematic overview of radiation therapy effects in soft tissue sarcomas,” Acta Oncologica, 2003; 42(5-6): pp. 516–531.
- Eric KS, Donald CM, Alaa M. Metastatic Soft Tissue Sarcomas: A Review Of Treatment and New Pharmacotherapies. P&T, 2018; 43(7).
- Seddon BM, Whelan J, Strauss SJ, et al. GeDDiS: A prospective randomised controlled phase 3 trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic STSs (EudraCT 2009-014907-29). J Clin Oncol 2015; 33(suppl): 10500.
- Yondelis (trabectedin) prescribing information. Horsham, Pennsylvania: Janssen Products, 2017.
- Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase 3 randomized multicenter clinical trial. J Clin Oncol, 2016; 34: 786–793.
- Votrient (pazopanib) prescribing information. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation, 2017; 47.
- Van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo controlled, phase 3 trial, 2012; 379(9829): 1879–1886.
- Stivarga (regorafenib) prescribing information. Whippany, New Jersey: Bayer HealthCare Pharmaceuticals, Inc, 2017.
- Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced STS (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol, 2016; 17: 1732–1742.

