A Novel Mutation in ARSA Gene Causing Metachromatic Leukodystrophy in a Saudi Boy, Genotype-Phenotype Correlation and Further Expansion of the Disease Spectrum
Talal AlAnzi* MD, Mohammed Asiri MD, Wejdan Hakami MD, Faisal AlEnezi MD
Prince Sultan Military Medical City, Pediatric Department, Clinical Genetics and Metabolic Division.
Prince Sultan Military Medical City, Pediatric Department.
Prince Sultan Military Medical City, Pediatric Department, Neurology Division.
Prince Sultan Military Medical City, Pediatric Residency.
Received Date: 17/10/2021; Published Date: 18/11/2021
*Corresponding author: Dr. Talal AlAnzi, Prince Sultan Military Medical City, Pediatric Department, Clinical Genetics and Metabolic Division, Saudi Arabia, Email: talanzi86@gmail.com
Abstract
Metachromatic leukodystrophy MLD is a hereditary neurometabolic disease affecting the myelin sheath that covers the nerves fibers in the central and peripheral nervous systems. MLD is caused by deficiency of the lysosomal enzyme arylsulfatase A (ARSA) or its activator protein namely Sposin B (SapB). The MLD patients clinically present with progressive psychomotor regression. ARSA and SapB protein deficiency ascribe to biallelic disease-causing mutations in the ARSA and PSAP genes, respectively. The MLD is severity variable and largely dependent on the residual activity of ARSA enzyme. The estimated prevalence of leukodystrophies in Saudi Arabia is 2.05/100,000 and male to female ratio is 1.5:1.
In this report, we describe a Saudi boy manifested with gross moto delay in the early infancy and the genetic analysis has yielded a ARSA variant of unknown significance. Using several methods based on clinical, biochemical and genetic evaluations, we concluded that ARSA variant is a pathogenic mutation.
Keywords: Metachromatic leukodystrophy; ARSA gene; Arylsulfatase a enzyme
Introduction
Metachromatic leukodystrophy MLD is a metabolic disorder of sulfatide storage in the nervous system and it is caused by deficiency of the lysosomal enzyme arylsulfatase A ARSA. It is a hereditary disease following the autosomal recessive pattern [1]. MLD term is derived from the observation of metachromatic granules in the affected tissues, made as a consequence of the accumulation of sulfatides and other complex lipid molecules in the myelin sheaths [2]
MLD is one of a wide range of genetic diseases causing abnormality in the brain white matter and it accounts nearly for a quarter of cases in a large multicentric Saudi cohort study [3].
MLD patients typically present mainly with progressive physical and cognitive decline, hypotonia, slurred speech and epilepsy. They could be categorized into three subtypes infantile, juvenile and adult forms depending on when the first manifestation starts [4].
Not only the enzyme deficiency is the single pathogenesis for MLD, the absence of a particular sphingolipid activator protein B (SapB) of ARSA enzyme can result in the same disorder [5].
Here, we report a 3½ -year-old boy from consanguineous Saudi parents with MLD diagnosed initially based on his clinical progressive psychomotor deterioration and MRI brain which revealed evidence of leukodystrophy. Whole exome sequencing yielded a homozygous variant of unknown significant in ARSA gene c.770A>G p. (Asp257Gly).
The family segregation analysis is in agreement with the clinical suspicion as the homozygosity was in the symptomatic index case. Arylsulfatase A enzyme assay confirmed that the variant is a disease-causing with its remarkably reduced level at Result 2.79 nmol/h*mg.
Case Report
Patient information
This is a 3½ -year-old male patient, full term, with a birth weight of 2.8 kg, uncomplicated spontaneous vertex delivery with no events afterwards. The patient was discharged with his mother on the next day. At the age of 1 month, the mother noticed right hand weakness. The patient was referred to orthopedics and plastic surgery with impression of radial club, grade 1 with hypoplastic hand.
The patient was referred to pediatrics neurology 2 years of age because of global developmental delay, saying only "baba" and "mama", sit with support, crawling with difficulty, and difficulty using the right hand. The mother also complains of episodes of choking mainly with sips of fluid with poor weight gain. Normal hearing and vision. No history of abnormal movement or seizure.
The parents are first cousins and they have 4 healthy kids. No similar history in the extended family.
Clinical findings
Upon examination at age of 3 years;
He was looking unwell, bound to a wheelchair, not in respiratory distress or pain. Vital signs were stable
- Chest: clear equal air entry bilaterally no wheezing and no crepitation.
- Cardiovascular: normal S1+S2 no murmur,
- Abdominal: Soft lax no tender no organomegaly
- Central nervous system: awake, GCS 14 /15, HC 48.5 cm between the 10th-25th Spastic diplegic with scissoring posture, following with eyes but with no head control, severe hypotonia, power3/5 bilaterally lower and upper limbs, with increased deep tendon reflexes all over.
Swallowing assessment was bedside by a swallowing pathologist, Impression: oropharyngeal dysphagia, he might be at risk of aspiration with thin fluids.
MRI brain: at 2 years, Figure A
Myelination is appropriate for the patient's age. Gray-white matter differentiation is preserved. Basal ganglia, thalami, brainstem and cerebellum are unremarkable. Ventricles and CSF spaces are normal.
There is no diffusion restriction or abnormal susceptibility signal is identified. MRS over the right basal ganglia demonstrates small lactate peak.
Inflammatory changes of paranasal sinuses are noted. Enlargement of adenoids with prominent bilateral retropharyngeal lymph nodes, which are likely reactive
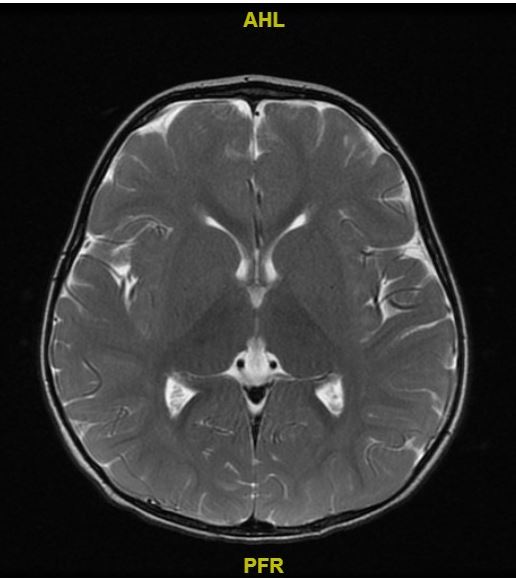
Figure A: MRI brain at 2 years showing normal grey-white matter differentiation.
CT brain: At 4 years, Figure B
There is supra and infratentorial mild white matter hypo attenuation for further evaluation by MRI. No acute intracranial hemorrhage. No mass effect and no space occupying lesion or hydrocephalus. The visualized osseous structures appear unremarkable.

Figure B: Diffuse white matter attenuation.
Therapeutic intervention
Underwent GT without fundoplication in at 3 years old
Following with physiotherapy to optimize his muscle strength.
MRI brain was rebooked at a later time.
Diagnostic assessment
Whole exome sequencing: A homozygous variant of uncertain significance in the ARSA gene c.770A>G p. (Asp257Gly).
The variant was validated by NGS.
- Variant coordinates: NM_000487.5: c.770A>G
- In silico parameters:
-Poly Phen: Probably damaging
-Align-GVGD: C0
-SIFT: Deleterious
-Mutation Taster: Disease
-Conservation amino acid: high
- Allele frequency: 0.05% in the Saudi Genome, seen twice as a heterozygous
- The variant is absent in homozygous state the genetic database such as OMIM, Pubmed , Varsome, Saudi Google scholar
- The family segregation analysis:
Father -/+ asymptomatic
Mother -/+ asymptomatic
Index case +/+ symptomatic
Sibling 1 male -/- asymptomatic
Sibling 2 female -/- asymptomatic
Sibling 3 female -/+ asymptomatic
Arylsulfatase A assay: blood leukocytes sample
2.79 nmol/h*mg
Reference range: --7 - 33 nmol/h*mg
The activity of Arylsulfatase A is below its reference range. This is in agreement with a classic metachromatic leukodystrophy (MLD)
Follow up and outcome
The patient was admitted at age of 2 years with severe tonsillitis with adenoid hypertrophy. He required PICU for intubation to secure the air way, CT scan of the neck showed: Inflamed palatine tonsils with small left tonsillar abscess.
ENT was involved and started on dexamethasone and antibiotics intravenously improved clinically over 48 and relocated to the general pediatric ward.
In the last visit 4 months ago, his spasticity has become more severe and lost his ability to fully control the head. Gastrostomy tube inserted because of high risk of aspiration.
He has attacks of convulsion at 3 years old. The CT brain showed diffuse white matter disease.
Discussion
Herein, we describe a 3½ Saudi boy with an infantile onset of metachromatic leukodystrophy. Although, the clinical assessment at the beginning could have raised MLD suspicion, the first brain MRI hasn’t shown the full-blown picture of the abnormal enhancements in the white matter. This would raise the necessity of repeating such an imaging study at later age to visualize the white matter changes at its early stages.
Furthermore, the availability of sophisticated molecular analyses like whole exome sequencing WES has aided us to reach the diagnosis in timely-manner. Nevertheless, WES could give inconclusive result as in our case, thus the utilization of adjunct biochemical marker study is a golden standard in such scenario.
Based on ACMG guidelines on variants classification published in 2015 [6] our gene variant c.770A>G p. (Asp257Gly) can be classified as pathogenic PS3.
Detection of pathogenic variants by WES is the most accurate way for diagnosing hereditary genetic and metabolic disorders. Yet, diagnosis can be made using other analyses (e.g., measuring of ARSA enzyme activity or sulfatide levels in the urine as well as MRI brain) since not all the pathogenic variants associated with MLD have been recognized or reported at the time being [7].
Therefore, we highlight the importance and necessity of utilizing the molecular methods for the diagnosis of the neurometabolic disorders.
Acknowledgment
To Centogene laboratory, Unilabs and Biocentia laboratories which have provided us with a very great assistance in doing the molecular as well as biochemical work up.
References
- Beerepoot S, van Dooren S, Salomons GS, Boelens JJ, Jacobs EH, van der Knaap MS, et al. Metachromatic leukodystrophy genotypes in The Netherlands reveal novel pathogenic ARSA variants in non-Caucasian patients. Neurogenetics, 2020; 21(4): 289-299.
- Shaimardanova AA, Chulpanova DS, Solovyeva VV, Mullagulova AI, Kitaeva KV, Allegrucci C, et al. Metachromatic Leukodystrophy: Diagnosis, Modeling, and Treatment Approaches. Frontiers in medicine, 2020; 7: 576221
- Alfadhel M, Almuqbil M, Al Mutairi F, et al. The Leukodystrophy Spectrum in Saudi Arabia: Epidemiological, Clinical, Radiological, and Genetic Data. Front Pediatr. 2021; 9: 633385.
- Harrington M, Whalley D, Twiss J, et al. Insights into the natural history of metachromatic leukodystrophy from interviews with caregivers. Orphanet J Rare Dis. 2019; 14(1): 89.
- Narayanan DL, Matta D, Gupta N, et al. Spectrum of ARSA variations in Asian Indian patients with Arylsulfatase A deficient metachromatic leukodystrophy. J Hum Genet. 2019; 64.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17.
- Dehghan Manshadi M, Kamalidehghan B, Aryani O, Khalili E, Dadgar S, Tondar M, et al. Four novel ARSA gene mutations with pathogenic impacts on metachromatic leukodystrophy: a bioinformatics approach to predict pathogenic mutations. Ther Clin Risk Manag, 2017.

