Intensity Modulated Radiotherapy of Two Simultaneous Neoplasms - Cervical Carcinoma and Breast Carcinoma
Vaska Vassileva, Lena Marinova*, Viktor Petrov
Medical Oncology Clinic, Radiation and metabolic brachytherapy Department, UMHAT “Queen Joanna” Sofia, Balgeria
Received Date: 11/10/2021; Published Date: 09/11/2021
*Corresponding author: Lena Marinova, Medical Oncology Clinic, Radiation and metabolic brachytherapy Department, UMHAT “Queen Joanna” Sofia, Balgeria
Summary
Concomitant expression of two neoplasms- Cervical Carcinoma (CC) and Breast Carcinoma (BC) is a relatively rare pathology. The manifestation of synchronous primary neoplasms is a challenge for the treating team as it puts a number of questions about the healing strategy.
We present a 57-year-old patient after total laparohisterectomy with lymphatic pelvic dissection on the local advanced CC/IIIB clinical stage. Intensity Modulated Radiotherapy (IMRT) in the small pelvis and the upper 2/3 of the vaginal cuff with Daily Dose (DD) 1.8 Gy up to Total Dose (TD) 50.4 Gy combined with Cisplatin (50 mg/m2) once a week, was conducted. After 4 months from the diagnosis and complex treatment of CC, PET/CT establishes a second neoplasm-invasive ductal carcinoma in the left mammary gland. After breast conserving surgery of BC, we are currently conducting Deep Inspiration Breath-Hold (DIBH) Radiation Technique on the left breast with DD 2 Gy up to TD 50 Gy. After 1 month of pelvic RT completion, RT on the paraaortal lymph nodes with DD 1.8 Gy up to TD 50 Gy should be conducted.
The discussion focuses on the simultaneous expression of two or more neoplasms, their relationship with genetic and other unfavorable predisposing factors, as well as the expected survival after the complex treatment of the two invasive carcinomas, involving IMRT.
For the treatment of multiple malignancies each case must be considered individually, ideally by a multidisciplinary team. If it is necessary to apply radiotherapy, the use of high-tech radiotherapeutic apparatus with the ability to perform modern radiant techniques such as IMRT is required.
Keywords: Synchronous primary neoplasms; Unfavorable predisposing factors; Prognosis; Complex treatment; Intensity modulated radiotherapy
Introduction
Diverticular disease occurs more frequently in the developed world and the aging demographics. It is prevalent in about 30% of individuals aged 60 and above, with slight preponderance in women between ages 50 and 70 years [1]. The sigmoid colon is involved in 95% of cases and this could become symptomatic in 70% of cases [1].
Diverticular disease could result in various life-threatening complications. These complications have been observed more in smokers, obese, and those who use non-steroidal anti-inflammatory drugs and low-fiber diets, and they include stricture, abscess, perforation, fistula, and bleeding. Though rare, strictures due to diverticular disease could cause complete bowel obstruction, in about 10% of large bowel obstructions [1].
Colorectal strictures are usually chronic and progressive. Though diverticular disease is the most common cause of benign colonic strictures, chronic diverticulitis is a rare cause of colon obstruction [2]. Only a few cases of colon strictures due to chronic diverticulitis has been reported, which poses a diagnostic challenge to differentiate from colon cancer, especially due to the varying clinical presentations. Hence the need for increased awareness for a high index of suspicion in the diagnosis of colonic strictures caused by diverticular disease in North America.
Clinical Case
A patient 57 years of age after radical laparohisterectomy with pelvic lymph node dissection on cervical carcinoma (IIIB clinical stage in FIGO, histological result - moderately differentiated (G2) squamous carcinoma, extensive necrosis areas, multiple tumor embolis in lymph and blood vessels, perineural invasion; isthmus and uterine body with massive infiltration from the abovementioned carcinoma; without serosis infiltration; 12 non-metastatic left pelvic lymph nodes, right pelvic lymph nodes-3 metastatic lymph nodes of 12 dissected lymph nodes. On 26.07.2021. the patient started postoperative Intensity Modulated Radiotherapy (IMRT) with VMAT technique in the pelvis and the upper 2/3 of the vaginal cuff with Daily Dose (DD) 1.8 Gy up to Total Dose (TD) 50.4 Gy combined with Cisplatin (50 mg / m2) once a week (Figure 1) The patient experienced very well postoperative simultaneous radio-chemotherapy (RT-Ch).
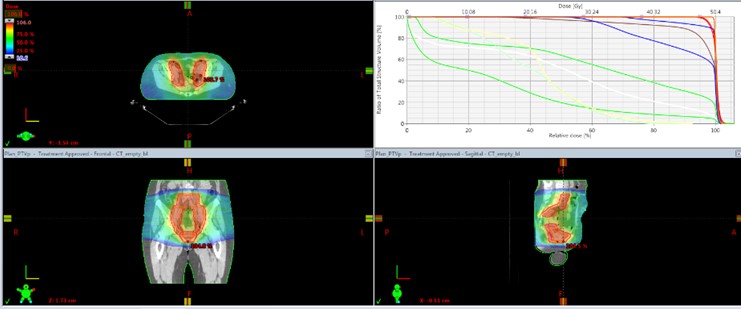
Figure 1: Postoperative intensity modulated radiotherapy (IMRT) with VMAT technique in the pelvis and the upper 2/3 of the vaginal cuff with Daily Dose (DD) 1.8 Gy up to Total Dose (TD) 50.4 Gy combined with Cisplatin (50 mg / m2) once a week.
After 4 months from the diagnosis and complex treatment of CC, PET/CT establishes a second neoplasm in the left mammary gland. Quadrantectomy with axillary lymph nodes dissection of I and II level with histological and immunohistochemical result moderately differentiated invasive ductal carcinoma (G2); tumor with a maximum diameter of 16 mm, 10 axillary lymph nodes without metastasis; estrogen receptor (ER) 2+; Progesterone Receptor (PR) negativ; HER2 (1+) negativ. After breast conserving surgery of BC, the left mammary gland was irradiated with Deep Inspiration Breath-Hold (DIBH) radiation technique by using volumetric modulated arc therapy (VMAT) with DD 2 Gy up to TD 50 Gy (Figure 2). After 1 month of pelvic RT completion, RT on the paraaortal lymph nodes with DD 1.8 Gy up to TD 50 Gy should be conducted .

Figure 2: Deep Inspiration Breath-hold (DIBH) radiation technique by using Volumetric Modulated Arc Therapy (VMAT) with DD 2 Gy up to TD 50 Gy.
The pelvic and left breast radiotherapy was conducted in weekly traceability of paraclinical indicators and strict monitoring of early radiation toxicity expression. The patient showed very good therapeutic tolerance without early radiation reactions from normal tissues and organs.
Discussion
In the 1930s Warren and Gates [5] proposed the first working definition of Multiple Primary Neoplasms (MPNs): (1) both tumors should be confirmed histologically as malignant; (2) each cancer must be anatomically separate and distinct; and (3) the second tumor must not be a recurrence or metastasis of the first cancer. This means that tumours arise in other organs than the independent primaries, each tumour has to be histologically distinctive and the possibility of metastasis or recurrence must be excluded [6,7]. The mechanism of development of MPNs is unclear and likely multifactorial; identified risk factors include previous cancer treatment, smoking, diet, and genetic mutations [8]. The synchronous expression of breast and cervical carcinoma is a rare event, due to various unfavorable predisposing factors [9]. With the active application of the PET/CT scanners in order to strictly define the stage of the malignant tumors, the possibilities for detecting synchronous neoplasms have risen sharply [10]. The appearance of synchronous primary neoplasms is a challenge for the treating team as it puts a number of questions about the patient's healing strategy. This is a therapeutic dilemma in everyday clinical practice [11]. Causal mechanisms of their development include the following: (i) host factors – genetic (BRCA mutations, Li-Fraumeni syndrome), hormonal, prior cancer diagnosis and treatment exposures, (ii) lifestyle factors such as alcohol and tobacco use (risk factors for several cancer types), and (iii) environmental influences – geography (areas of increased radon exposure), pathogens (human papilloma virus or Epstein-Barr virus infections) and occupational factors [12-17]. Patients with multiple primaries have less aggressive malignancies, present at earlier stages, frequently have a strong family history of similar cancer, and their cancers tend to have indolent clinical behavior with longer survival rates, possibly related to genetic predisposition [14]. Synchronous genital tract neoplasms are rare but cause more clinical problems than a single neoplasm [18,19]. Interesting clinical case with synchronous cervical carcinomas (exocervix squamous cell carcinoma and the clear cell endocervix adenocarcinoma), accounting for Human Papilloma Virus (HPV) 18, which was detected in the squamous cell carcinoma; however, without HPV in the clear cell adenocarcinoma [20]. 4-17% of patients with Breast Cancer (BC) develop multiple primary neoplasias. The most frequent type of synchronous malignancy in patients with BC is contralateral breast cancer (63.3% of cases) followed by female genital organ cancer (13.4% of cases) [6]. In the publication of L. Marinova et al. / 2015, an interesting and extremely rare clinical case of two synchronous tumors in the breast, namely invasive ductal carcinoma and primary breast osteosarcoma [21]. Most often diagnosed synchronous neoplasms are contralateral breast cancer and genital cancers, of which most common is cervical cancer [22]. Genetic factors (e.g., BRCA1, BRCA2) are seen as habitual risk factors for multiple primary tumors [23]. Published data indicate that age as well as menopausal status at breast cancer diagnosis are risk factors for development of second cancer [24-26]. Studies in Japan and Italy estimated that 9%-11% of early gastrointestinal carcinomas develop other malignancies [27,28]. Five-year survival rates were higher for metachronous cancers (95%) than for synchronous primaries (59%) and single primaries (59%) [14]. For the treatment of multiple malignancies, each case must be considered individually, ideally by a multidisciplinary team, accounting for the type and stage of each tumor, response to treatment, and the patient's overall health status [8]. In the present case, the familial history of the patient implies a high risk of malignant neoplasms as her mother had uterine cancer. Breast cancer is a socially significant, most commonly diagnosed neoplasm in women. In the current clinical case, as it is a I clinical stage BC after a conservative breast surgery, no chemotherapy is required. For stage IIA ВС, the decision was to treat her with chemotherapy followed by postmastectomy radiation therapy due to multiple risk factors, including focal lymphovascular space invasion and 2 positive lymph nodes [29]. Breast-preserving surgery with subsequent high-tech radiotherapy has a great contribution to achieving very good therapeutic and cosmetic results [30]. Adjuvant Left Breast Radiotherapy (ALBRT) for breast cancer can result in significant radiation dose to the heart [31]. As the number of BCs survivors has increased, chronic sequelae of breast cancer RT has become more important [32]. Heart-sparing can be performed in three different ways in breast cancer radiotherapy: by seeking to keep the heart out of treated volumes (i.e. by prone position or specific breathing techniques such as Deep Inspiration Breath-Hold (DIBH) and/or gating), or by using modern radiation techniques like IMRT, Volumetric Modulated Arc Therapy (VMAT) or protons [33]. Deep Inspiration Breath-hold (DIBH) is a well-established radiation technique to achieve a significant cardiac dose reduction during adjuvant Radiotherapy (RT) in left-sided breast cancer (Figure 2). A new linac system with an integrated Surface Scanner (SS) for DIBH treatments could easily be incorporated into daily routine and is associated with significant dose reduction to the heart and ipsilateral lung [34].
Conclusion
Concomitant expression of two neoplasms- Cervical Carcinoma (CC) and Breast Carcinoma (BC) is a relatively rare pathology. The manifestation of synchronous primary neoplasms is a challenge for the treating team as it puts a number of questions about the healing strategy. The forecast is dependent on the histological results of the corresponding primary tumors, accounting for the type and stage of each tumor and the adequate complex treatment. For the treatment of multiple malignancies, each case must be considered individually, ideally by a multidisciplinary team. If it is necessary to apply radiotherapy, the use of high-tech radiotherapeutic apparatus with the ability to perform modern radiant techniques such as IMRT is required.
References
- Nina A Mayr, William Small Jr, David K Gaffney. Decision Making in Radiation Oncology, 2011; 2.
- Scott Bermudez R, Kim Huang, I-Chow Hsu. Handbook of Evidence-Based Radiation Oncology; Chapter 29, 2010.
- Bulgarian National Cancer Register, University Specialized Hospital for Active Oncology Treatment, Tom XXVI, 2020.
- Casey W. Williamson, Anthony Paravati, Majid Ghassemi, еt al. Five Simultaneous Primary Tumors in a Single Patient: A Case Report and Review of the Literature. Case Rep Oncol. 2015; 8(3): 432-438.
- Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932; 16: 1358–1414.
- Lee J, Park S, Kim S, et al. Characteristics and survival of breast cancer patients with multiple synchronous or metachronous primary cancers. Yansei Medical J, 2015; 56: 1213–1220.
- Filali K, Hedelin G, Schaffer P et al. Multiple primary cancers and estimation of the incidence rates and trends. Eur J Cancer, 1996; 32A: 683-690.
- Coleman MP. Multiple primary malignant neoplasms in England and Wales, 1971-1981. Yale J Biol Med. 1986; 59: 517-531.
- Sharma DN, Chander S, Awasthy BS, Rath GK. Synchronous occurrence of carcinoma of the uterine cervix and of the breast. Eur J Surg Oncol. 1999; 25: 547–548.
- Amol Padegaonkar, Pranav Chadha, and Archana Shetty. A Rare Case of Synchronous Cervical and Breast Carcinoma. Indian Journal of Surgical Oncology. 2018; 9(4): 622–623.
- Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res, 2014; 6: 119-134.
- 12. Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev, 2006; 15: 2020–2026.
- Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open, 2017; 2: e000172.
- Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res, 2014; 6: 119–134.
- Mariotto AB, Rowland JH, Ries LA et al. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev, 2007; 16: 566–571.
- Calip GS, Law EH, Ko NY. Racial and ethnic differences in risk of second primary cancers among breast cancer survivors. Breast Cancer Res Treat 2015; 151: 687-696.
- Molina-Montes E, Requena M, Sanchez-Cantalejo E et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol, 2015; 136: 158–171.
- Cheng-Kuo Lin, Mu-Hsien Yu, Ta-Wei Chu, Hung-Cheng Lai. Synchronous occurrence of primary neoplasms in the uterus with squamous cell carcinoma of the cervix and adenocarcinoma of the endometrium. Taiwan J Obstet Gynecol. 2006; 45(4): 336-339.
- Y Yasui , K Suzumori, Y Yagami, T Nakamura. Squamous cell carcinoma of the endometrium coexistent with synchronous adenocarcinoma of the cervix. Gan No Rinsho. 1987; 33(15): 1954-1958.
- Gynecol Oncol, 2005; 97(3): 976-979.
- Kenji Goto , Yuzuru Takeuchi, Akira Yakihara, Fumikazu Kotsuji. Synchronous invasive squamous cell carcinoma and clear cell adenocarcinoma of the uterine cervix: a different human papillomavirus status. Gynecol Oncol. 2005; 97(3): 976-979.
- Lena Marinova, Tatyana Hadjieva, Emil Kanchev, and Svetla Vicheva. Synchronous primary mammary osteosarcoma and invasive breast cancer. A case report – Pathohistological and immunohistochemical analysis. Rep Pract Oncol Radiother. 2015; 20(1): 72–76.
- Beata Sas-Korczyńska, Jerzy W. Mituś, Wojciech Kamzol, et al. Synchronous malignancies in patients with breast cancer. NOWOTWORY J Oncol, 2017; 67: 336–341.
- VogtA, SchmidS, HeinimannK, et al. (2017) Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2(2):e000172.
- Schaapveld M, Visser O, Louwman MJ et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol, 2008; 26: 1239–1246.
- Chen Y, Thompson W, Semenciw R, et al. Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev, 1999; 8: 855–861.
- Hislop TG, Elwood JM, Coldman AJ et al. Second primary cancer of the breast: incidence and risk factors. Br J Cancer, 1984; 49: 79–85.
- Ikeguchi M, Ohfuji S, Oka A, et al. Synchronous and metachronous primary malignancies in organs other than the stomach in patients with early gastric cancer. Hepatogastroenterology, 1995; 42: 672–676.
- Bozzetti F, Bonfanti G, Mariani L, et al. Early gastric cancer: unrecognized indicator of multiple malignancies. World J Surgery, 2000; 24: 583–587.
- Ebctcg. McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8,135 women in 22 randomised trials. Lancet, 2014; 383: 2127–2135.
- Marinova L, Petrova K, Evgeniev N. Radiotherapy after Breast-Preserving Surgery for Early Breast Cancer-Basic Principles and Our Experience. Austin J Med Oncol. 2021; 8(1): 1058.
- Amy J Hayden , Melissa Rains, Kenneth Tiver. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol. 2012; 56(4): 464-472.
- Chirag Shah, Shahed Badiyan, Sameer Berry, et al. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol. 2014; 112(1): 9-16.
- 33. Marciana-Nona Duma , René Baumann , Wilfried Budach, et al. Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO) Strahlenther Onkol. 2019; 195(10): 861-871.
- 34. Rodrigo Hepp, Mark Ammerpohl, Christina Morgenstern, et al. Deep inspiration breath-hold (DIBH) radiotherapy in left-sided breast cancer: Dosimetrical comparison and clinical feasibility in 20 patients. Strahlenther Onkol. 2015; 191(9): 710-716.

