An Unusual Case of Simultaneous Left and Right Sided Infective Endocarditis: A Report of a Coronary Cameral Fistula
Vivek S Narayan Pillai*, Sajeev CG, Kadermuneer P, Sajeer K, Ranjith MP, Rajesh KF, Mohanan Sandeep, Rajesh Gopalan Nair and Krishnan MN
Department of Cardiology, GMC Kannur, India
Received Date: 10/10/2021; Published Date: 03/11/2021
*Corresponding author: Pillai Shankar Narayan Vivek, MD (General Medicine), DM (Cardiology), PDF (Cardiac Electrophysiology), Assistant Professor Cardiology and Electrophysiology, GMC, Kannur-670503
Abstract
Simultaneous involvement of left and right sided valves in infective endocarditis is rare [1]. Infective endocarditis, although reported as complication of underlying pathologic processes and anatomic abnormalities, is not well substantiated. We report a case of simultaneous involvement of left and right sided valves in a patient with coronary cameral fistula.
Introduction
Simultaneous multivalvular involvement in infective endocarditis is uncommon [1]. Predisposing conditions include ruptured sinus of valsalva1 dental extraction, burns and implantable prosthetic devices [2-4]. Literature regarding the clinical course, pathophysiology and prognosis of infective endocarditis involving both left and right sided valves is scanty. Our patient had a coronary cameral fistula from right coronary artery to right atrium, predisposing her to simultaneous left and right sided infective endocarditis.
Case Report
A 47-year-old female was referred to our department with 6 months intermittent fever and NYHA functional class 2 dyspnea. She was previously evaluated elsewhere for pyrexia of unknown origin, and empirically started on anti-tuberculous therapy. On presentation she was febrile with temperature of 100F. General examination revealed pallor and clubbing. Cardiovascular examination revealed cardiomegaly suggestive of left ventricular enlargement.
The second heart sound was single and loud. A blowing early diastolic murmur decrescendo in quality was present. Laboratory investigation revealed a hemoglobin of 8.5 gm%, elevated ESR of 105 and C –Reactive Protein of 146 mg/L. Peripheral smear revealed microcytic hypochromic anemia and workup for connective tissue disease was negative. Blood culture grew alpha- hemolytic streptococci, sensitive to vancomycin, gentamycin and linezolid. ECG revealed her to be in sinus rhythm, with a rate of 110/ mt. and normal QRS axis. Chest X-ray showed cardiomegaly with prominence of the main pulmonary artery and obliteration of the pulmonary bay. Transthoracic and transesophageal echocardiogram revealed a 10 x 6 mm vegetation on the right coronary cusp of the aortic valve with significant aortic regurgitation (Figure 1(A)), tricuspid valve vegetation of size 11x 14 mm Figure 1(E) and, a coronary fistula from the right coronary artery to the right atrium (Figure 1(B)-1(D)), and a 26x 18 mm vegetation in the fistulous connection from the right coronary artery to the right atrium, Figure 1(E). The right coronary artery was found to be dilated at its origin from the aortic sinus Figure 1(F). In addition, she was found to have severe pulmonary hypertension with right ventricular systolic pressure of 50 mm Hg. In order to study the abnormal cardiac anatomy, the patient was subjected to a cardiac CT, which revealed a fistulous communication between the sinoatrial nodal branch of the right coronary artery and the right atrium Figure 2(A), as well as pulmonary thromboembolism in the left descending pulmonary artery, with pulmonary infarcts. The patient was treated with parenteral vancomycin and gentamycin for a period of 30 days, and was afebrile at the time of discharge, along with normalization of inflammatory markers.
Follow up echocardiogram showed diminution in the size of the vegetations. She underwent an aortogram and coronary angiogram ,which showed the fistulous communication from the right coronary artery to the right atrium Figure 2(B).She successfully underwent correction of the coronary cameral fistula as well as aortic valve replacement.
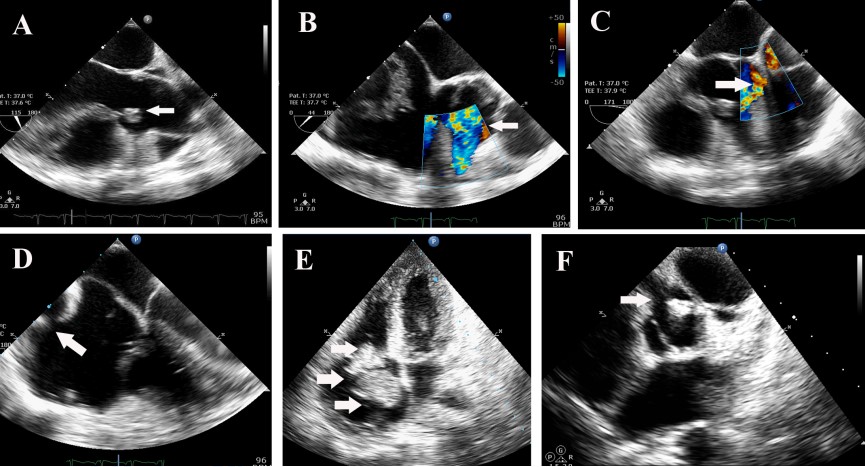
FIGURE 1A: Transesophageal echo- arrow showing aortic valve vegetation.
FIGURE 1B and FIGURE 1C: Transesophageal echo- colour Doppler, arrow showing flow in the coronary fistula.
FIGURE 1D-Transesophageal echo showing entry of the coronary fistula into dilated right atrium. (vertical arrow).
FIGURE 1E-Transthoracic echo -4 chamber view showing tricuspid valve vegetation (top horizontal arrow), and echogenic mass in the right atrium (lower horizontal arrow).
FIGURE 1F-Transthoracic echo modified short axis view, showing dilated origin of right coronary artery (top arrow), and aortic valve vegetation (lower arrow).
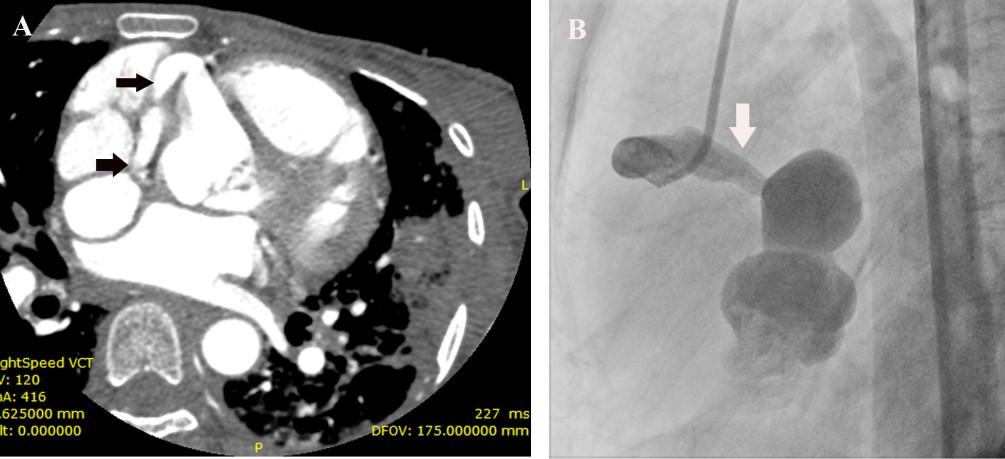
FIGURE 2A- CT angiogram showing a dilated vessel arising from the right coronary artery (arrow) and communication with an aneurysmal sac.
FIGURE 2B-Coronary angiogram showing dilated right coronary artery emptying into an aneurysmal sac.
Discussion
Simultaneous multisite infective endocarditis is a rarity, and the cases reported result from causes as varying as a ruptured sinus of valsalva1, infective endocarditis of aortic bioprosthetic valve with concomitant tricuspid valve involvement2. The common factor in all these cases was an immunocompromised state, and additionally, in the case of the ruptured sinus of Valsalva, an anatomic passage facilitating the involvement of the right sided valves. Involvement of the right sided valves can further cause dissemination of infection to the pulmonary vasculature as occurred in our patient, as well as cause pulmonary embolism. This report highlights the importance of appropriate antibiotic therapy in such cases, all the previous cases of simultaneous multisite endocarditis were treated with appropriate antibiotics, but ran very aggressive courses, in part due to the organism (staphylococcus in the majority), which has the reputation for rapid progression of infection and high mortality [1,4].
References
1. Kadir S, Burgos H, Hanoman HH. Ruptured Sinus of Valsalva complicated by right and left sided endocarditis, R →L - shunt and congestive cardiac failure. West Indian med. j. 2010; 59(2).
2. Van der zee, Van Bergen, Dekkers, et al. Two cases of left-sided and concomitant right-sided endocarditis: potential pathways of spreading.Neth Heart J, 2012; 20: 472–474.
3. Huang, Pontes, Slome. Multisite vancomycin–intermediate sensitive staphylocoocus aureus infective endocarditis with giant vegetations on implantable cardioverter defibrillator lead. Circulation. 2011; 123: 457-458.
4) Mak, Milliken, Saremi. Multisite infective endocarditis with mural vegetations in the right atrium and right ventricle. Circulation. 2009; 119: 2643-2644.

