Decreased Heart Rate Variability in COVID-19
Chengfen Yin1,2, Jianguo Li3, Zhiyong Wang2, Yongle Zhi2, Lei Xu*2,
1The Third Central Clinical College of Tianjin Medical University, China
2Department of Critical Care Medicine, Tianjin Third Central Hospital, Tianjin Key Laboratory of Extracorporeal Life Support for Critical Diseases, Artificial Cell Engineering Technology, Research Center, Tianjin Institute of Hepatobiliary Disease, China
3Department of respiratory and critical medicine, Tianjin Haihe hospital, China
Received Date: 20/09/2021; Published Date: 18/10/2021
*Corresponding author: Lei Xu, Department of Critical Care Medicine, Tianjin Third Central Hospital, Artificial Cells Key, Laboratory of Tianjin, Tianjin Institute of Hepatobiliary Disease, Artificial Cell Engineering, Technology Research Center of Public Health Ministry, Tianjin 300170, China
Abstract
On March 12, 2020, the World Health Organization (WHO) announced that the coronavirus disease 2019 (COVID-19) outbreak had become a pandemic. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which primarily infects the lower airways and binds to Angiotensin Converting Enzyme 2 (ACE2) on alveolar epithelial cells. ACE2 is widely expressed, not only in the lungs but also in the cardiovascular system. Therefore, SARS-CoV-2 can also damage the myocardium. We analyzed three COVID-19 cases that resulted in death, and found that either COVID-19 or antiviral drugs could affect coupling between the autonomic nervous system and the sinus node, thus affecting heart rate variability and preventing the heart rate from rising in response to increases in body temperature. Early detection of the preclinical phase of cardiac autonomic dysfunction may help determine patients in need of aggressive treatment and control of cardiovascular risk factors. Antiviral drugs should be used with caution in patients with heart injury.
Introduction
In December 2019, an outbreak of a novel coronavirus, now formally named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, Hubei province, central China. SARS-CoV-2 causes coronavirus disease 2019 (COVID-19), and the World Health Organization (WHO) has declared COVID-19 a global public health emergency [1]. Although reported case numbers in China are slowing, large outbreaks in other countries, including Italy, the United States, Iran, and South Korea, are of great concern. On March 12, 2020, the WHO officially announced that the COVID-19 outbreak was a pandemic [2].
Information about the new coronavirus and its health impact is constantly being updated. SARS-Cov-2 predominantly infects the lower airways and binds to Angiotensin Converting Enzyme 2(ACE2) on alveolar epithelial cells. SARS-Cov-2 viruse is potent inducer of inflammatory cytokines. The virus activates immune cells and induces the secretion of inflammatory cytokines and chemokines into pulmonary vascular endothelial cells. The postulated mechanism for the resultant organ damage is the “cytokine storm” or “cytokine cascade” response [3]. In addition to lung damage, COVID-19 can cause varying degrees of damage to the heart, kidney, liver [4,5]. ACE2 is widely expressed in the human body, not only in the lungs but also in the cardiovascular system, and thus SARS-CoV-2 can also damage the myocardium. However, the specific mechanisms of cardiovascular damage are unclear. Patients with COVID-19 who have an underlying cardiovascular disease have an adverse prognosis4. Therefore, early detection of cardiovascular damage by COVID-19 is important, and particular attention should be given to cardiovascular protection in the treatment of COVID-19 [6].
In this report, we present three COVID-19 cases that resulted in death. The immediate cause of death was cardiogenic shock in one patient and cardiac arrest in two patients. We found that either COVID-19 or antiviral drugs could affect coupling between the autonomic nervous system and the sinus node, thus affecting heart rate variability and preventing the heart rate from rising in response to increases in body temperature. The aim of this report is to highlight cardiac injury caused by SARS-CoV-2. Decreased HRV could be used to distinguish cardiac injury in COVID-19 earlier than myocardial markers. Increased awareness of decreased HRV can lead to improved treatment and patient prognosis.
Case Presentation
This study was approved by the National Health Commission of China and Ethics Commission of Tianjin Third Central Hospital (2020-03-14). Written informed consent was waived by the Ethics Commission of the hospital.
As of March 14, 2020, a total of 136 patients had been diagnosed with COVID-19 in Tianjin, China. Three (2.2%) of these patients died in the early stage of the COVID-19 epidemic. The demographic and clinical characteristics of these cases are shown in Table 1. The remaining 131 (96.32%) patients recovered and were discharged from the hospital; of these, 2 (1.47%) patients had remained in the hospital after being discharged from the intensive care unit.
On admission, the degree of severity of COVID-19 was categorized as common type in Case 1 and Case 3, and as severe type in Case 2. All three cases were positive for SARS-CoV-2 based on Polymerase Chain Reaction (PCR) test results on admission. All three cases had at least one comorbidity (e.g., hypertension, diabetes mellitus, and cardiovascular disease). All three cases had varying degrees of cough and fever on admission, and all three cases had bilateral lung ground-glass opacity on computed tomography imaging. Case 2 presented with respiratory distress (respiratory rate, 36 breaths/min). Case 1 and Case 3 presented with respiratory rates within the normal limit. On admission, Case 1, Case 2, and Case 3 had, respectively, a heart rate of 88 bpm, 94 bpm, and 76 bpm; mean arterial pressure of 100 mmHg, 94 mmHg, and 110 mmHg; SpO2 of 100%, 98%, and 100%; Q-SOFA score of 0, 1, and 1; and APACHE II score of 5, 15, and 11.
The laboratory test results of the three cases on admission are listed in Table 2. Liver and renal functions of all three cases were almost normal on admission. The blood gas and electrolyte results indicated that the internal environment was in a stable state. Case 1 and Case 3 had elevated levels of C-reactive protein, indicating that both patients were in an inflammatory state. The procalcitonin levels of the three cases were within normal limits.
All three cases were diagnosed and treated according to Pneumonitis Diagnosis and Treatment Program for New Coronavirus Infection (Trial Version 5) [7]. The antiviral therapy used for all three cases was ritonavir plus interferon-α (as shown in Table 1). The immediate cause of death for Case 1, Case 2, and Case 3 was cardiogenic shock (hospital stay, 2 days), cardiac arrest (hospital stay, 8 days), and cardiac arrest (hospital stay, 9 days), respectively (Table 1).
The cardiac biomarkers of the three cases are shown in Table 3. COVID-19–related cardiac injury can be diagnosed if serum levels of cardiac biomarkers (e.g., troponin I) are above the 99th percentile upper reference limit [4]. Troponin I and CK-MB were increased substantially only in Case 3, for whom the diagnosis of virus-related cardiac injury could not be made until day 7. Myoglobin levels were above the normal limit in Case 1 and Case 2 on admission. Myoglobin levels were above the normal limit in Case 3 from day 5 to day 9. NT-proBNP levels were above the normal value in Case 1, but within the normal value in Case 2 and Case 3 for the duration of the hospital stay.
Changes in heart rate and body temperature are presented in Table 4. Generally, heart rate has a specific relationship with body temperature. As body temperature increases, the heart rate will also increase. Recent studies have shown that, in acutely admitted patients, heart rate increases by 7.2 times/min when body temperature increases by 1ºC [8]. After adjusting for age, oxygen saturation, and mean blood pressure, the heart rate will increase by 6.4 times/min when body temperature increases by 1 degree [8]. Based on this theory, the heart rates of Case 1, Case 2, and Case 3 should have increased by 8 times/min, 17 times/min, and 13 times/min, respectively, as their temperature rose; however, the actual increases in heart rate were 5 times/min, 13 times/min, and 4 times/min, respectively. The lower increases in heart rate than what would theoretically be expected indicates a decrease in the patients’ heart rate variability (HRV). Decreases in HRV occurred earlier in all three cases than increases in cardiac biomarkers (e.g., troponin I and CK-MB).
Table 1: Demographic and clinical characteristics of the three cases.
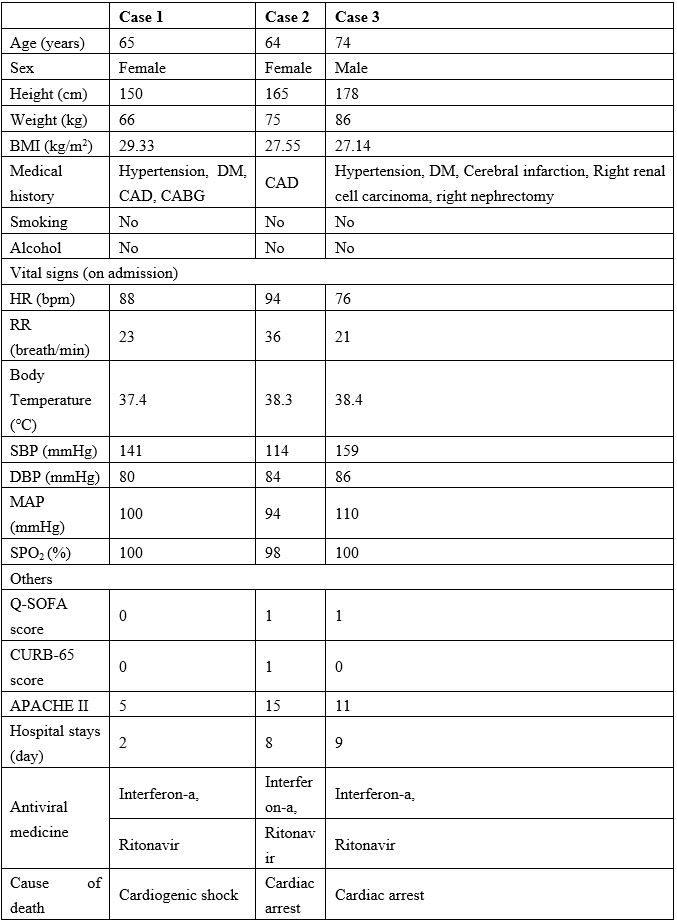
Note: BMI=body mass index, DM=diabetes mellitus, CAD=Cardiovascular disease, CABG=Coronary artery bypass surgery, HR=Heart rate, RR=Respiratory frequency, SBP=Systolic blood pressure, DBP=Diastolic blood pressure, Q-SOFA score=Quick-sequential organ failure assessment, APACHE II=Acute physiological and chronic health status evaluation II.
Table 2: Laboratory results of the three cases on admission.
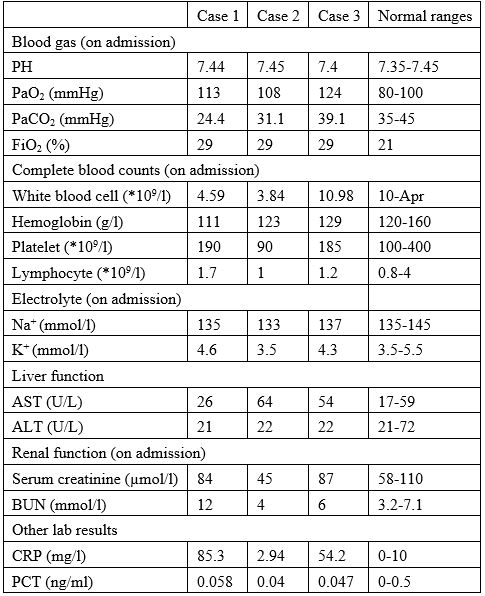
Note: PaO2=Arterial oxygen partial pressure, PaCO2=Arterial partial pressure of carbon dioxide,
FiO2=fraction of inspired oxygen, Na+=Sodium ion, K+=Potassium ion, AST=Aspartate aminotransferase, ALT=Alanine aminotransferase, BUN=Blood urea nitrogen, CRP=C-reactive protein, PCT=Procalcitonin.
Table 3: Cardiac markers of the three cases.
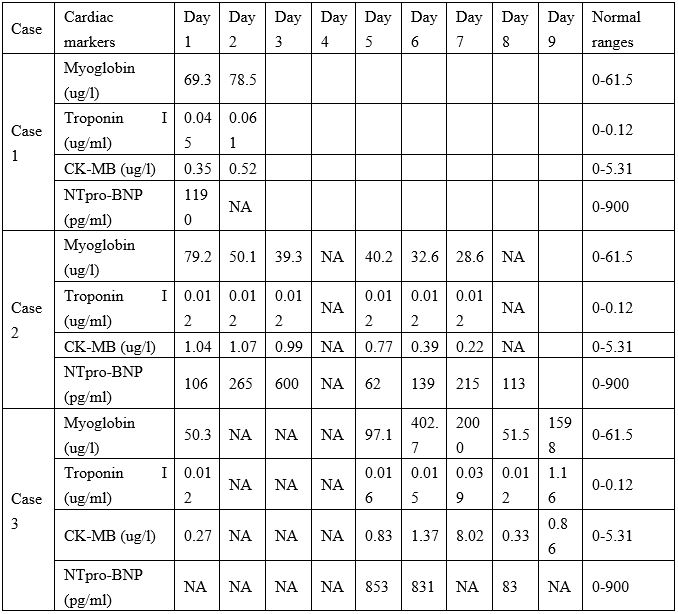
Note: NA=not available
Table 4: Changes in heart rate and body temperature of the three cases.
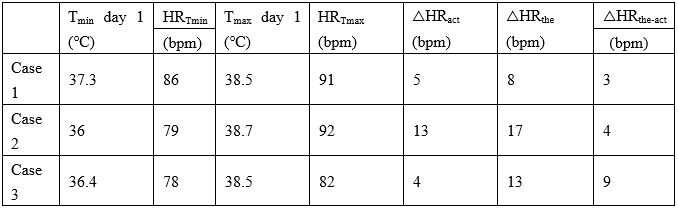
Note: Tmin=Minimum body temperature, HRTmin=Heart rate at the minimum body temperature, Tmax=Maximum body temperature, HRTmax=Heart rate at the maximum body temperature, △HRact=The actual increase in heart rate as the body temperature increases, △HRthe=Theoretical increase in heart rate as body temperature increases,
△HRthe-act=The difference between the theoretical and actual increase of heart rate.
Discussion
Although the clinical manifestations of COVID-19 are dominated by respiratory symptoms, some patients have severe cardiovascular damage [4]. In addition, some patients with underlying cardiovascular diseases might have an increased mortality risk [4]. Therefore, understanding the damage caused by SARS-CoV-2 to the cardiovascular system and the underlying mechanisms is of great importance, allowing for the timely and effective treatment of these patients.
ACE2 has been identified as a functional receptor for coronaviruses [9], including SARS-CoV-2 and SARS-CoV. ACE2 is expressed not only in the lungs but also in the cardiovascular system. Thus, ACE2-related signaling pathways might also play a role in heart injury. Other proposed mechanisms of myocardial injury include a cytokine storm triggered by an imbalanced response of Type 1 and Type 2 T helper cells [4,10], or damage to myocardial cells from respiratory dysfunction and hypoxemia caused by COVID-19. Previous data show that older patients (aged > 60 years) who are infected with SARS-CoV-2 tend to have more systemic symptoms and more severe pneumonia than younger patients (aged ≤60 years) [11]. Therefore, in patients with COVID-19, underlying cardiovascular disease can exacerbate the pneumonia and increase the severity of disease.
Generally, heart rate has a specific relationship with body temperature. As body temperature increases, heart rate will also increase [8]. However, increases in the body temperature of the three present cases did not correspond with appropriate increases in heart rate. This indicates reduced HRV, which could be due to a problem in coupling between the autonomic nervous system and the sinus node. Autonomic nervous system (ANS) imbalance, with a shift toward decreased vagal and increased sympathetic tone, has been proven to be associated with higher risk of cardiac mortality. The presence of autonomic dysfunction should alert clinicians to the possibility of coexisting cardiovascular risk factors. Early detection of the preclinical phase of cardiac autonomic dysfunction may lead to the implementation of more aggressive treatment and control of cardiovascular risk factors. Some data indicate that autonomic imbalance can be related to an increased risk of arrhythmias, and even sudden future death [12].
The sinus node is the natural pacemaker of the heart and possesses its own intrinsic activity; nevertheless, a variety of external and internal stimuli that change the balance between vagal and sympathetic tone influence the final basic heart rate. Heart rate changes may occur as a response to physical or mental stress, cardiac or noncardiac diseases, or pharmacological or invasive treatment. HRV describes variations in both instantaneous heart rate and RR intervals. HRV is considered an indirect measure of autonomic regulation of cardiac activity, and can reflect the coupling between the ANS and the sinoatrial node [13]. Studying the physiological signals (eg. heart rate) of critically ill patients can easily identify “hidden” information concerning inherent dynamics and overall variability within a time series [14]. Therefore, HRV has become an important and well-recognized tool in identifying patients at risk of cardiogenic death [12]. Time-domain–based HRV parameters decreased in patients may reflect higher risk of future mortality [12]. In the three present cases, decreases in HRV occurred earlier than did changes in cardiac markers. In future similar cases, staying alert to early changes in HRV may help to predict the occurrence of cardiac events in the early stage.
The reasons for reduced HRV in infectious disease still debated. The first theory focuses on reduction of vagal tone [15]. The second theory states that normal physiology has fractal-like properties with high levels of complexity, which explains phenomena such as HRV [16]. Severe disease reflects a “decomplexification” that can mainly be attributed to uncoupling between different restorative mechanisms [17]. Accumulating evidence, including human studies in cardiac transplant recipients with hearts devoid of autonomic nervous inputs, supports a potential third mechanism associated with an intracardiac origin of HRV, which was first proposed by Griffin et al [18]. According to that hypothesis, sinus node cells with an extreme heterogeneity in electrophysiological properties and intercellular connections of sinus node cells can be viewed as an amplifier of various input signals [19]. In infectious or cardiovascular diseases, an unfavorable metabolic milieu could affect ion channel gating properties or membrane receptor densities, with significant impact on the level and variability of pacemaker activity. In addition, a possible reduced responsiveness of sinus node cells to external stimuli could also negatively affect HRV [18].
At present, there are no specific antiviral drugs or vaccines against COVID-19 infection approved for therapeutic use in humans. Broad-spectrum antiviral drugs are the only available option, such as nucleoside analogues and HIV-protease inhibitors (lopinavir/ritonavir) that can attenuate virus infection until a specific antiviral becomes available [20]. All three of the present cases were treated with ritonavir. However, two studies of combination antiretroviral therapy used for patients with HIV reported that autonomic dysfunction was present in the treated patients [21,22]. Such autonomic dysfunction may be due to neuropathy-related adverse effects of antiretroviral therapy [23,24,25]. Moreover, the effect of combination antiretroviral therapy on metabolic disturbances could also lead to parasympathetic damage [26]. Many antiviral drugs can cause cardiac insufficiency, arrhythmia, or other cardiovascular disorders. Therefore, during the treatment of COVID-19, which necessitates the use of antivirals, the risk of cardiac toxicity must be closely monitored [27].
The clinical findings of these three cases suggest that either the mechanisms of COVID-19 and/or the antiviral drugs used for treatment might affect coupling between the autonomic nervous system and the sinus node, thus affecting HRV and inhibiting increases in heart rate as body temperature increases. The use of a 24-hour Holter monitor is recommended in the management of COVID-19 to better monitor heart rate. Early detection of the preclinical phase of cardiac autonomic dysfunction could indicate the need for aggressive treatment and control of cardiovascular risk factors, thus improving patient prognosis. Antiviral drugs should be used with caution in patients with heart injury.
Acknowledgement
We thank all other members of Tianjin Third Central hospital and Tianjin Haihe hospital, resilience and creativity in responding to the COVID-19 crisis.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Funding Support
This study was supported by Rui E (RuiYi) special fund for emergency medical research (grant number R2019006); Science and technology planning project of Tianjin (grant number
18ZXDBSY00100), and Tianjin Natural Science Foundation Project (grant number 19JCQNJC10000).
Role of Funder
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- World Health Organization. Coronavirus disease (COVID-19) outbreak.
- Who announces Covid-19 outbreak a pandemic.
- Jiang F, Deng L, Zhang L, et al. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19), J Gen Intern Med. 2020. DOI: 10.1007/s11606-020-05762-w.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223): 497-506. DOI: 10.1016/S0140-6736(20)30183-5.
- Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua Gan Zang Bing Za Zhi. 2020; 28(2): 148-152. DOI: 10.3760/cma.j.issn.1007-3418.2020.02.003.
- Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system.Nat Rev Cardiol. 2020. DOI: 10.1038/s41569-020-0360-5.
- https://news.youth.cn/jsxw/202002/t20200205_12186073.html.
- Jensen MM, Kellett JG, Hallas P. Fever increases heart rate and respiratory rate; a prospective observational study of acutely admitted medical patients.Acute Med. 2019; 18(3): 141-143.
- Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004; 25(6): 291-294. DOI: 10.1016/j.tips.2004.04.001.
- Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004; 136(1): 95-103. DOI: 10.1111/j.1365-2249.2004.02415.x.
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020; 395(10223): 514-523. DOI: 10.1016/S0140-6736(20)30154-9.
- Iwona Cygankiewicz, Wojciech Zareba,Heart rate variability, Handbook of Clinical Neurology, Vol. 117 (3rd series),Chapter 31: 379-393.
- Papaioannou VE, Verkerk AO, Amin AS, et al. Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev. 2013; 9(1): 82-96. DOI: 10.2174/157340313805076359.
- Seely AJE, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems. Crit Care Med 2000; 28: 2193-2200. DOI: 10.1097/00003246-200007000-00003.
- Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981; 213(4504): 220-222. DOI: 10.1126/science.6166045.
- Goldberger AL, Amaral LAN, Hausdorff JM, et al. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci USA 2002; 99: 2466-2472. DOI: 10.1073/pnas.012579499.
- Godin PJ, Buchman TG. Uncoupling of biological oscillators: A complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 1996; 24: 1107-1116. DOI: 10.1097/00003246-199607000-00008.
- Griffin MP, Lake DE, Bissonette EA, et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 2005; 116 (5): 1070-1074. DOI: 10.1542/peds.2004-2461.
- Zaza A, Lombardi F. Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovsc Res 2001; 50: 434-442. DOI: 10.1016/s0008-6363(01)00240-1.
- H Lu. Drug treatment options for the 2019-new coronavirus (2019-nCoV), Biosci. Trends (2020), Biosci Trends. 2020; 14(1): 69-71. DOI: 10.5582/bst.2020.01020.
- Lebech AM, Kristoffersen US, Mehlsen J, et al. Autonomic dysfunction in HIV patients on antiretroviral therapy: studies of heart rate variability. Clin Physiol Funct Imaging. 2007; 27(6): 363-367. DOI: 10.1111/j.1475-097X.2007.00760.x.
- Askgaard G, Kristoffersen US, Mehlsen J, et al. Decreased heart rate variability in HIV positive patients receiving antiretroviral therapy: Importance of blood glucose and cholesterol. PLoS One. 2011; 6(5): e20196. DOI: 10.1371/journal.pone.0020196.
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs.J Peripher Nerv Syst. 2001; 6(1): 14-20. DOI: 10.1046/j.1529-8027.2001.006001014.x.
- Peltier AC, Russell JW. Recent advances in drug-induced neuropathies. Curr Opin Neurol. 2002;15(5): 633-638. DOI:10.1097/00019052-200210000-00015.
- Authier FJ, Gheradi RK. Peripheral neuropathies in HIV-infected patients in the era of HAART. Brain Pathol. 2003; 13(2): 223-228. DOI: 10.1111/j.1750-3639.2003.tb00021.x
- Licht CM, Vreeburg SA, van Reedt Dortland AK, et al. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab 2010; 95: 2458-2466. DOI: 10.1210/jc.2009-2801.
- Sakabe M, Yoshioka R, Fujiki A. et al. Sick sinus syndrome induced by interferon and ribavirin therapy in a patient with chronic hepatitis C. J Cardiol Cases. 2013,29; 8(6): 173-175. DOI: 10.1016/j.jccase.2013.08.002.

