Retroperitoneal Duodenal Perforation Secondary to Early Plastic Stent Migration: Case Report and Literature Review
Pereda D, Gonzalo R*, Gallego T, Bolinaga I, Ruiz P, Loidi O, Bustillo I, JM Gutiérrez-Cabezas
Department of General Surgery, Sierrallana Hospital, Spain
Received Date: 14/09/2021; Published Date: 06/10/2021
*Corresponding author:Rubén Gonzalo González, Department of General Surgery, Sierrallana Hospital, Ganzo s/n, 39300, Torrelavega (Cantabria), Spain
Abstract
Endoscopic biliary stenting for biliary decompression is a well-established treatment for patients with obstructive jaundice due to biliary stricture. Few plastics biliary stents migrate (5–14%), but in some cases they can cause serious complications. Duodenal perforation is a rare but potentially fatal complication. Management remains controversial, but early diagnosis and treatment are key because a delay of 24 hours or more has a negative impact on survival and quality of life. Treatment options vary according to the situation: it can be used conservative antibiotic treatment, endoscopic treatment and in some cases, surgery is required. We present a case of an older female patient with a retroperitoneal perforation at the duodenum secondary to plastic biliary endoprostheses migration who required an emergency surgery.
Keywords: Biliary obstruction; Endoprostheses; Complications; Migrated biliary stent; Bowel perforation
Introduction
Endoscopic biliary stenting for biliary decompression is a popular, sometimes first-line, treatment for patients with obstructive jaundice due to biliary stricture. Intestinal perforation secondary to stent migration is a rare but potentially fatal complication. A high degree of diagnostic suspicion is key for early treatment. We present the case of a retroperitoneal duodenal perforation secondary to plastic biliary stent migration in a patient with biliary stricture who was treated at a tertiary hospital.
Case Report
An 85-year-old female patient had a history of cervical fracture limiting daily activity. She suffered from recurrent cholangitis secondary to choledocholithiasis. After two inconclusive endoscopic retrograde cholangiopancreatographies (ERCPs), a 9-cm plastic biliary stent was placed during a third ERCP. The procedure was initially uneventful. Eight hours after the procedure, the patient presented at the emergency department with intense widespread abdominal pain and no further symptoms. She had jaundice with a total bilirubin level of 15.9. At exploration, the abdomen was rigid. Blood tests showed increased cholestatic and PCR enzymes, as well as coagulation disorders, without leukocytosis or left deviation. A CT scan revealed intrahepatic and extrahepatic biliary tract dilatation, with pneumobilia and presence of a biliary stent whose distal end was located extraluminally (Figure 1). The gallbladder had little fluid inside and hyperdense heterogeneous content.
An emergency exploratory laparotomy was performed. It showed a retroperitoneal perforation at the third portion of the duodenum, with the plastic stent protruding through it (Figure 2). In addition, the gallbladder was atrophic, and the common bile duct was indurated and swollen. The stent was removed, and the duodenum was sutured. The biliary tract was explored and no choledocholithiasis was detected. A Kehr’s T tube with approximately 200 ml of bile flow was placed in the common bile duct.
Postoperative evolution was slow, and the patient was discharged 2 weeks later. She was re-admitted 1 week later with fever and jaundice despite installation of a permeable Kehr’s T tube. Antibiotic treatment was initiated. A magnetic resonance cholangiopancreatography was conducted and showed an image suggestive of main biliary tract neoformation (Klatskin tumor), 3.2 cm in diameter, causing intrahepatic biliary tree dilatation.
The patient’s situation and therapeutic and palliative options were discussed with the family. The decision was made to limit therapeutic effort. The patient eventually passed away.
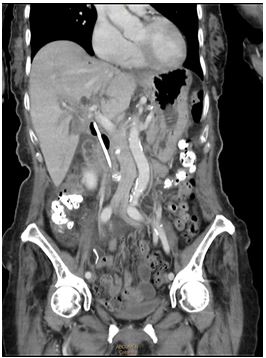
Figure 1: Contrast-enhanced abdominal pelvic CT-scan: Duodenal perforation in a patient with biliary stent. Pneumoperitoneum, free fluid, and subhepatic collection. Intrahepatic and extrahepatic biliary tract dilatation.
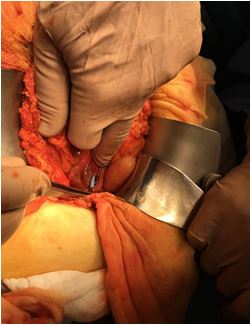
Figure 2: Retroperitoneal duodenal perforation with the plastic stent protruding through it.
Discussion
Endoscopic drainage of the biliary tract is a well-established treatment both in cases of benign and malignant strictures, either temporary or permanent. Metallic stents are typically used in malignant strictures as a permanent solution, whereas plastic stents are used mostly in benign strictures or as a temporary preoperative procedure, though this varies according to patient needs and medical issues. Stent placement is not free from complications. In the short term, bleeding, pancreatitis, cholangitis, and intestinal perforation may occur, with subsequent occlusion, stent migration, and potential intestinal perforation [1].
Approximately 5–14% of plastic biliary stents migrate [1–3]. Distal migration is more frequent than proximal migration (5.9% vs. 4.9%, respectively) [4]. Most stents pass through the small bowel without causing any symptoms. In less than 1% of cases, they cause hollow viscera perforation [3,5]. The duodenum is the most common perforation site in this extremely rare complication (92%),6 with a poor prognosis and high mortality rate (50%) [50], perhaps because it has a thinner wall than the rest of the small bowel [3,7]. Stents occasionally migrate distally and reach the small bowel or colon, where they cause complications, especially in patients with diverticulosis, previous abdominal surgery, or hernia [1,2,8]. In these patients, plastic stents could be contraindicated [4,6]. Retroperitoneal perforations are more frequent than intraperitoneal perforations, but the latter are more severe [5,7]. Migration can occur during the procedure, days or even weeks later, but it is extremely rare in the first hours [5].
Stent migration risk factors include presence of benign biliary stricture, use of a plastic stent, placement of a single stent, and < 3 months since procedure [5,7–9]. In addition, shorter stents tend to migrate proximally, whereas longer ones usually migrate distally [5]. In our case study, the patient experienced distal migration within 8 hours of placing a single, long, plastic stent. A large angle (≥30º) between the distal side of the stent and the central line of the patient’s body is another risk factor of perforation due to distal stent migration [7].
Clinical signs are highly variable. Some patients experience no symptoms or mild discomfort, some present with abscesses in retroperitoneal perforations, and some experience acute abdomen with pain, fever, nausea, vomiting, and other symptoms [2,5,10]. Because these symptoms also occur frequently in patients with pancreatitis following ERCP, early differential diagnosis is key to avoid treatment delays. Laboratory findings are highly unspecific, though amylase levels and liver function testing could show increases [9]. Consequently, imaging tests prove highly useful for diagnostic purposes. Various classifications of duodenal perforation following ERCP have been suggested. Stapfer et al. established four types in descending order of severity, according to production mechanism and perforation location and including treatment implications [13].
Ultrasonography has been proven helpful in terms of diagnosis and assessing retroperitoneal air removal in conservatively treated patients [10]. However, contrast-enhanced abdominal CT scan remains the diagnostic tool of choice because it detects very low volumes of intraperitoneal and retroperitoneal air and fluid [11,12]. Additionally, in patients with a retained stent and no symptoms, CT scan allows monitoring of progress and potential complications [8]. Furthermore, simple X-ray shows normal results in about 50% of perforation cases [10].
All patients undergoing biliary stent placement require close follow-up [2]. Advances in interventional radiology, endoscopy, and laparoscopy have improved treatment scope and reduced treatment morbidity in perforation secondary to biliary stent migration therapies, both for conservative and surgical treatments [14]. Management remains controversial, but early diagnosis and treatment are key. A delay of 24 hours or more has a negative impact on survival and quality of life [11,14]. Treatment options vary according to patient condition, perforation size and type, and the physician in charge [3].
Conservative antibiotic treatment with bowel rest and parenteral nutrition may be attempted in more stable patients [9], in patients with symptom-free retroperitoneal perforations, in elderly patients with multiple comorbidities, or in the presence of advanced malignant neoplasia [10]. A comprehensive description of patients that may benefit from this treatment is key [9]. For smaller perforations that are endoscopically accessible, stent removal and intestinal wall defect closure with clips or loops is the treatment of choice [12,15]. However, in cases of endoscopic treatment failure, diagnostic hesitation, unstable patients, or patients with clinical signs of acute abdomen or radiological evidence of widespread peritonitis, surgery is required [1]. Surgical options include stent removal and primary closure alone, defect closure with drainage and abdominal cavity lavage, and in severe cases with significant peritonitis and intestinal wall inflammation, pyloric exclusion [10].
In our case, due to the difficulty of previous ERCP attempts and the clinical signs of acute abdomen, a surgical treatment was selected. Considering the early diagnosis achieved through abdominal CT scan and the patient’s age and quality of life, a simple duodenal suture sufficed.
Conclusion
Duodenal perforation due to biliary stent migration is a rare but potentially fatal complication. Any clinical symptom occurring in the first hours following stent placement should be considered suggestive of a more severe complication and necessitates quick action. Contrast-enhanced abdominal CT scan is the imaging test of choice. Treatments are multiple and remain under discussion. Early detection and action are key, either through endoscopy or emergency surgery in unstable patients or with radiological evidence of peritonitis. In some instances, suturing the perforation will suffice, but in more severe or advanced cases, pyloric exclusion may be required.
Author Contributions
1. Toribio Gallego: examined and operated the patient
2. Deiane Pereda: drafted manuscript and performed an additional literature
3. Rubén Gonzalo: edited and revised manuscript
All the authors contributed to, read and approved the final version of the manuscript.
Conflicts of Interest/Competing Interests
No competing interests.
Grant Information
The authors received no specific funding for this work.
Acknowledgements
Not applicable.
References
- Yilmaz Ö, Kiziltan R, Aydin O, Bayrak V, Kotan Ç. A rare complication of biliary stent migration: small bowel perforation in a patient with incisional hernia. Case Rep Surg. 2015; 2015: 860286.
- Namdar T, Raffel AM, Topp SA, Namdar L, Alldinger I, Schmitt M, et al. Complications and treatment of migrated biliary endoprostheses: a review of the literature. World J Gastroenterol. 2007; 13(40): 5397-5399.
- Ferm S, Fisher C, Hassam A, Rubin M, Kim SH, Hussain SA. Primary endoscopic closure of duodenal perforation secondary to biliary stent migration: a case report and review of the literature. J Investig Med High Impact Case Rep. 2018; 6: 2324709618792031.
- Yaprak M, Mesci A, Colak T, Yildirim B. Biliary stent migration with duodenal perforation. Eurasian J Med. 2008; 40(3): 154-156.
- Jadallah K, Alzubi B, Sweidan A, Almanasra AR. Intraperitoneal duodenal perforation secondary to early migration of biliary stent: closure with through-the-scope clip. BMJ Case Rep. 2019; 12(9): 230324.
- Akimboye F, Lloyd T, Hobson S, Garcea G. Migration of endoscopic biliary stent and small bowel perforation within an incisional hernia. Surg Laparosc Endosc Percutan Tech. 2006; 16(1): 39-40.
- Yuan XL, Ye LS, Liu Q, Wu CC, Liu W, Zeng XH, et al. Risk factors for distal migration of biliary plastic stents and related duodenal injury. Surg Endosc. 2020; 34(4): 1722-1728.
- Tao Y, Long J. Sigmoid colon perforation caused by migrated plastic biliary stents: a case report. Int J Colorectal Dis. 2020.
- Thapa N, Loudin M, Sharzehi K, de Melo SW Jr, Enestvedt BK. Endoscopic or conservative management of iatrogenic duodenal perforations caused by long plastic biliary stent distal migration. ACG Case Rep J. 2020; 7(7): 00430.
- Miller G, Yim D, Macari M, Harris M, Shamamian P. Retroperitoneal perforation of the duodenum from biliary stent erosion. Curr Surg. 2005; 62(5): 512-515.
- Bharathi RS, Rao PP, Ghosh K. Intra-peritoneal duodenal perforation caused by delayed migration of endobiliary stent: a case report. Int J Surg. 2008; 6(6): 478-480.
- Wang X, Qu J, Li K. Duodenal perforations secondary to a migrated biliary plastic stent successfully treated by endoscope: case-report and review of the literature. BMC Gastroenterol. 2020; 20(1): 149.
- Langerth A, Isaksson B, Karlson BM, Urdzik J, Linder S. ERCP-related perforations: a population-based study of incidence, mortality, and risk factors. Surg Endosc. 2020; 34(5): 1939-1947.
- Saranga Bharathi R, Rao P, Ghosh K. Iatrogenic duodenal perforations caused by endoscopic biliary stenting and stent migration: an update. Endoscopy. 2006; 38(12): 1271-1274.
- Bureau MA, Gkolfakis P, Blero D, Pezzullo M, Devière J, Lemmers A. Lateral duodenal wall perforation due to plastic biliary stent migration: a case series of endoscopic closure. Endosc Int Open. 2020; 8(5): 573-577.

