Acute Ischemic Stroke Following Covid-19 Vaccination
Houda Mokhlis*
Department of Medicine, Mohammed V University, Morocco
Received Date: 10/09/2021; Published Date: 01/10/2021
*Corresponding author:Houda Mokhlis, Department of Medicine, Mohammed V University, Rabat, Morocco
Introduction
SARS-CoV-2 spread rapidly across the world causing the COVID-19 pandemic, the agent behind the coronavirus disease is a single-stranded RNA virus of the coronaviridae family; it is a respiratory pathogen that emerged in late 2019 [1]. Several clinical trials are currently underway in search of therapies for COVID-19.
After the release of covid-19 vaccines; mortality and hospitalisation rates for fully vaccinated people seem to decrease, however, like other vaccines, it is not without side effects.
Case Report
We report the case of 46 years old female patient mother of two, with no cardiovascular risk factors, who presented to the emergency room for a sudden left sided hemiparesis with central facial palsy seven days following the second dose of covid-19 vaccine.
The initial brain scan performed was unremarkable, the EKG and the cardiovascular examination were normal with a NIHSS score of 14, in the absence of medical contraindication the patient received thrombolytic treatment with Tenecteplase, a control scan was performed the next day showing a hypodense area of the right superficial middle cerebral artery territory with signs of haemorrhagic transformation;

Figure 1: cerebral scan images showing the stroke.
The evolution was marked by the installation four days later of a right monocular blindness, with a stage 1 papilledema at the fundus examination.
Initial blood tests showed no abnormalities except for an iron-deficient microcytic hypochromic anaemia and a D-dimer elevation with a normal platelet count.
An immunological assessment including the search for anti-native DNA, B2GP1, CARDIOLIPIN antibodies, and ANCA (Antineutrophil cytoplasmic antibodies) was also performed; and returned negative.
In the framework of the etiological assessment, a trans-thoracic echography was performed, which came back normal, as well as a supra-aortic trunk echo-doppler, that showed a discreet atheromatous overload with the presence of a fresh mobile thrombus with complete obstruction of the right internal carotid artery.
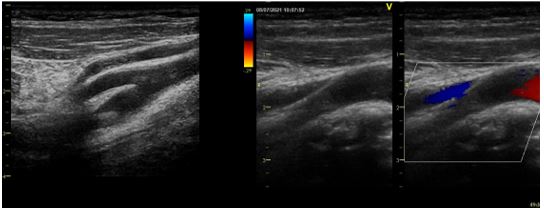
Figure 2: Echodoppler of the supra-aortic trunks (2D and color mode) objectifying a thrombus of the proximal part of the right internal carotid artery floating at the bulb.
Discussion
COVID-19 vaccines have been introduced to control the pandemic, adverse effects such as fatigue, local reactions at the injection site, fever, myalgia and muscle pain, headache were frequent, however less common and more serious side effects like acute disseminated encephalomyelitis and large vessel stroke were also linked to covid-19 vaccines.
Immune thrombocytopenia triggered by vaccination with cerebral venous thrombosis (CVT) is a syndrome recently described in young adults within two weeks from the first dose of the vaccine.
“Vaccine-induced immune thrombotic thrombocytopenia” (VITT or TTS) results from the development of immune mediated thrombocytopenia by platelet-activating antibodies against platelet factor 4 (PF4) [2,3]. Several reports documented this finding among mRNA vaccines recipients [4], even though the majority of the reported cases of VITT are Cerebral Venous Thrombosis, arterial thrombosis in the brain or in other organs should not be excluded.
Our patient didn’t have any other risk factors such as atrial fibrillation, diabetes and atherosclerosis so the search of an uncommon cause of thrombosis was necessary; VITT should be suspected if the platelet count is lower than <150x109/L and D‐dimers are elevated or Fibrinogen levels are reduced , in fact patients presenting an ischaemic stroke after receiving the Covid-19 vaccine should be evaluated by laboratory tests including platelet count, D-dimers, fibrinogen and anti-PF4 antibodies as well as search for co-existing venous thromboses.
Our patient had only one criterion for VITT (Markedly elevated D-dimer)
However, A patient who presents with thrombosis and a normal platelet count post-vaccination might be in an early stage of TTS (Thrombotic Thrombocytopenia Syndrome); that’s why a Continued assessment for development of thrombocytopenia is required [5].
Conclusion
The COVID-19 pandemic is a worldwide public health concern with many consequences for human health and well-being. COVID-19 vaccines are considered safe since the proven benefits of vaccination in protecting against COVID-19 outweighs the risks.
Nonetheless, health professionals must be aware of all possible complications, early diagnosis and quick initiation of the appropriate treatment may enhance the outcome.
References
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents 2020; 55.
- Nazy I, Sachs UJ, Arnold DM, McKenzie SE, Atlhaus P, Choi K. Recommendations for the clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) for SARS-CoV-2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemostasis 2021.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Eichinger P, Kyrle S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination, 2021.
- Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al.: Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol 2021.
- James B Bussel, Jean M Connors, Douglas B Cines, Cynthia E Dunbar, Laura C Michaelis, Lisa Baumann Kreuziger, et al. Thrombosis with Thrombocytopenia Syndrome; AMERICAN SOCIETY OF HEMATOLOGY)

