The Prophylactic Treatment of COVID-19 in A Renal Failure and Hodgkin Lymphoma Patient with Convalescent Plasma
Mehmet Ali Erkurt*
Department of Medicine, Inonu Unıversıty, Turkey
Received Date: 15/08/2021; Published Date: 07/09/2021
*Corresponding author: Mehmet Ali Erkurt, Department of Medicine, Inonu Unıversıty, Turkey
Abstract
COVID-19 is a viral infectious disease that is thought to have emerged from an animal market in Wuhan, People's Republic of China, in December 2019, and subsequently caused a pandemic. Despite the fact that 1 year has passed since the onset of the epidemic and many treatment agents have been tried, there is still no approved treatment. Convalescent plasma therapy is a passive immunization method and holds promise in the treatment of COVID-19. In this study, we observed that asymptomatic COVID-19 infection was effectively treated with convalescent plasma in a young patient with a diagnosis of Hodgkin lymphoma, acute renal failure also who received R-DHAP (Rituximab – Cisplatin – Cytarabine-Dexamethasone) chemotherapy treatment. We think that this treatment may be more effective when it started in earlier stage of disease in asymptomatic patients.
Keywords: Covid 19; Lymphoma; Renal Failure; Convalescent Plasma
Introduction
In the last 2 decades, infections belonging to the corona virus family have led to outbreaks. From them, SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome) caused fear in 2002 and 2012 but the main pandemic was SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona virus 2) who is a new member of the corona virus family. He made it under the name of COVID-19 [1].
Convalescent plasma therapy is a passive immunization method which an effective and reliable treatment was used more than 100 years for various infectious disease and holds promise in the treatment of COVID-19 [2]. Because of the lack of B- and T-cell-derived immunity, immunosuppressed patients may benefit more from convalescent plasma treatment [3]. Here, we report the rapid and effective treatment of COVID-19 disease with convalescent plasma that develops in our patient with Hodgkin lymphoma who infected shortly after her chemotherapy.
Despite the fact that 1 year has passed since the onset of the epidemic and many treatment agents have been tried, there is still no approved treatment [4]. Although the difficulties in treating immunocompetent individuals still remains, also effort in developing treatment modality continues in immunosuppressed individuals, especially in patients with hematological malignancies.
Case Report
A 22-year-old female patient received 6 cycles of ABVD (Adriamycin-Bleomycin-Vinblastin-Dacarbazine) treatment with the diagnosis of Hodgkin Lymphoma 2 years ago. She was followed up in remission until to October 2020. R-DHAP (Rituximab – Cisplatin – Cytarabine-Dexamethasone) chemotherapy was started due to recurrence in the patient. In the second cycle of the treatment, drug-induced nephrotoxicity developed in the patient and she received hemodialysis and renal replacement therapy for 6 times. She was referred to our clinic for hematopoietic stem cell transplantation. She was admitted to our service and the COVID PCR test was sent from the nasopharyngeal swab sample in accordance with our transplant protocol. Because of the positivity of her test, Favipravir treatment was initiated to the asymptomatic patient. Serum immunoglobulin levels measured and IgA deficiency did not detected. COVID convalescent plasma therapy was administered. First, antibody titer of the convalescent plasma donor was measured. Plasma was collected from the donor with an IgG level above 1/320.200 cc plasma was administered in 15 minutes and there was no complication. The patient was followed up in the COVID ward. Renal replacement therapy of the patient was continued in this place. In the follow-up, intravenous meropenem 3x1000 mg / day treatment was started. On the 7th day of the convalescent plasma treatment, the COVID PCR test which was taken from the nasopharyngeal swab sample became negative. It is planned to continue autologous stem cell transplantation procedure after recovery of kidney function. The table below shows the patient's results before and after convalescent plasma.
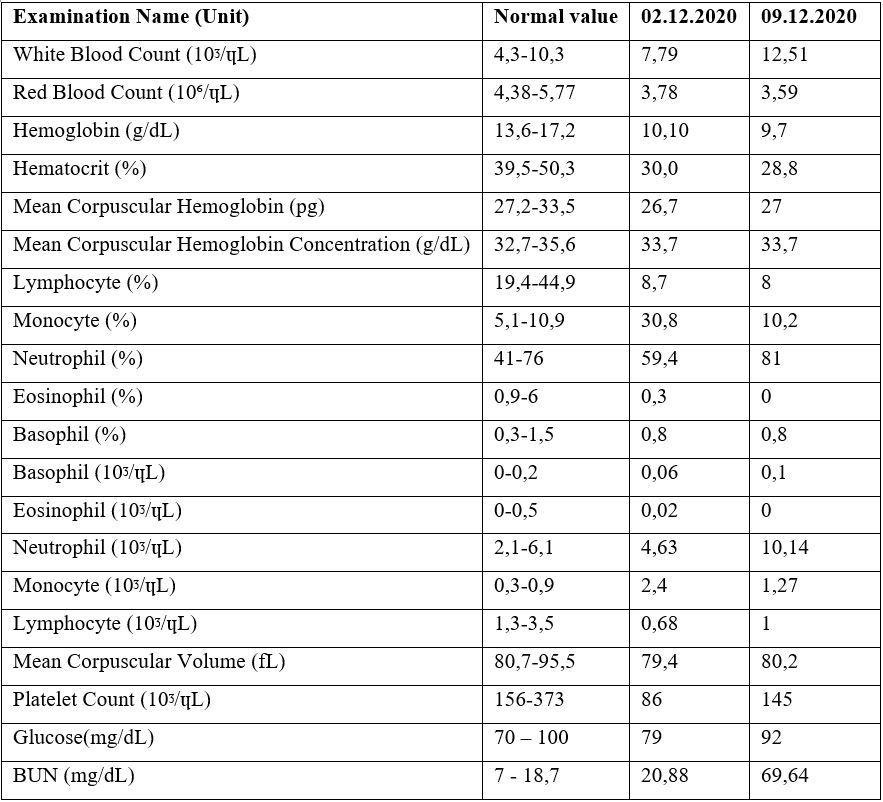
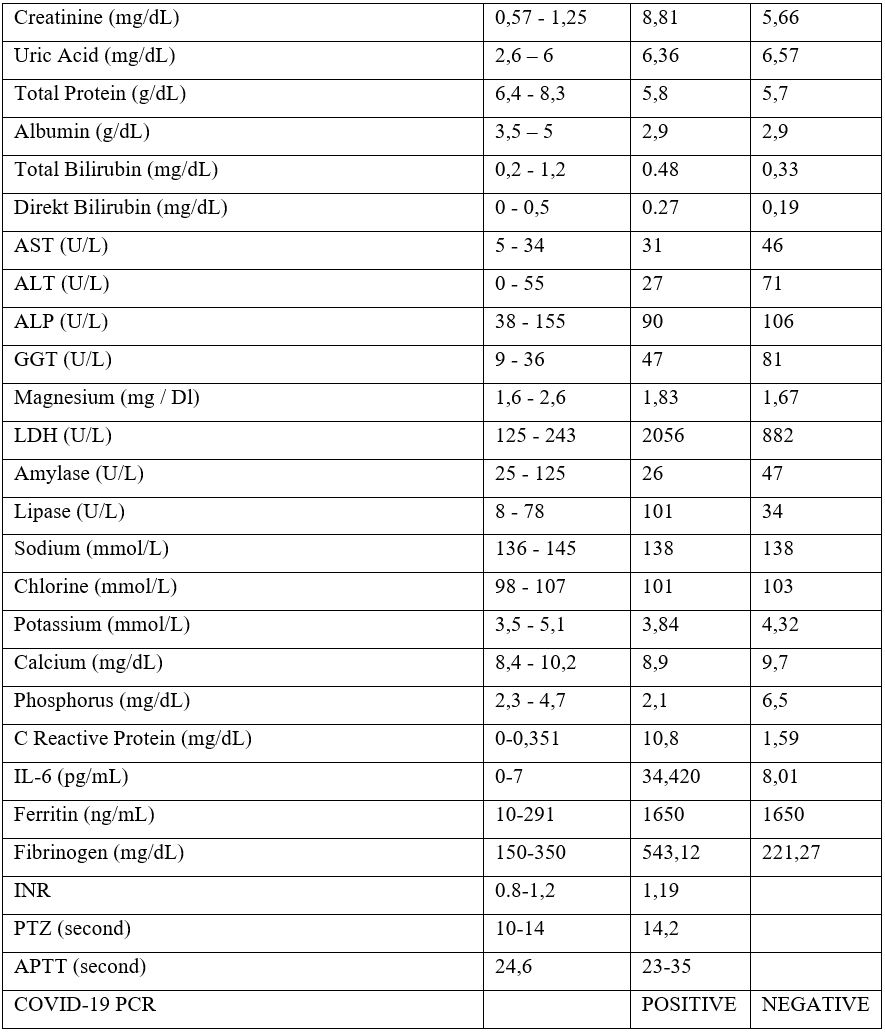
Discussion and Conclusion
The treatment of COVID-19 in immunosuppressed patients is still a mystery. Increased mortality and morbidity are an expected result in patients with hematological malignancies and solid organ transplantation [3]. It is obvious that in patients with hematological malignancies, both innate and humoral immunity weaken due to the primary disease and the agents used in disease treatment. Currently, there is no standard treatment for COVID-19. The agents used are mostly antiviral drugs such as favipravir, remdesivir, lopinavir-ritonavir, oseltamivir. Immune convalescent plasma therapy is a promising treatment in this respect. The use of convalescent plasma is recommended in patients with solid organ transplantation, hematological malignancy and low immunity [5]. Adding to this, it is advised that antiviral therapy and convalescent plasma treatments are used in combination [6]. When the current literature is reviewed, it has been observed that convalescent therapy is mostly used in patients with advanced stage, such as acute respiratory distress syndrome [7,8]. Data on the use of convalescent plasma in the treatment of hematological cancer patients with COVID-19 infection is very limited. However, it has been reported as an effective and safe treatment [5]. Similar to our case, convalescent plasma can be used as a safer treatment in patients with comorbidities such as kidney failure and in which not all drugs can be used.
In a study of 17 patients receiving anti-B-cell treatments, it was reported that the effectiveness of convalescent plasma treatment emerged in a short period of 1 week [9]. In the result of Senefeld’s work, within 48 hours of convalescent plasma transfusion the majority of 54 patients who have hematological malignancies demonstrated improved clinical status and viral clearance [10]. In addition, it was reported that the convalescent plasma administered in a non-Hodgkin Lymphoma patient who was seronegative and whose complaints continued even 88 days of viral meeting. After the onset of plasma his complaints relieved immediately [11]. In another case, convalescent plasma was transfused a patient who was on the day 20 of hospitalization. Following this treatment, the patient became independent of ECMO within one day and was successfully weaned from mechanical ventilation within two days [12]. In addition, a report of 16 patients in USA says steady improvement in oxygenation levels was observed following each convalescent plasma infusion. All of the 5 intubated patients were extubated between 1- and 19-days post convalescent plasma infusion. The remaining 11 showed a dramatic decline in oxygen needs and did not require ventilatory support [13].
We observed that asymptomatic COVID-19 infection was effectively treated with convalescent plasma in a young patient with Hodgkin lymphoma, acute renal failure and who received anti CD-20 therapy. As stated in some studies in the literature, we saw that our patient turned negative for the COVID-19 PCR test within 1 week [14]. When it was given inside of the 72 hours of admission, convelesant plasma recipients <65 years had 4-fold lower mortality and 4-fold lower deterioration in oxygenation on severe Covid-19 patients [15]. In another 3 studies, it was stated that when convalescent plasma is used at an average of 44 and 72 hours, it reduces mortality on the elderlypatients. It should be used in the early periodespecially before cytokine storm begins [16-18]. In the meta-analysis of 17 studies, 5 of which were randomized, it was reported that the mortality rates of patients who received convalescent plasma were significantly lower than those who did not [19]. FDA (United States Food and Drug Administration) announces best outcomes occur in thosewho receive a high-titer unit of convalescent plasma within 3 days of COVID-19 diagnosis or admission to the hospital [20]. We think that the clinical improvement of the patient situation may be due to other drugs used in combination with convalescent plasma. Convalescent plasma can be used as an additive therapy agent in such patients where there is no standard treatment and the mortality rate is high due to the underlying disease. Finally, this treatment may be more effective in asymptomatic andimmunosuppressed patients when it is given as soon as they are diagnosed.
References
- Dhama K, Khan S, Tiwari R, et al. Coronavirus Disease 2019-COVID-19. ClinMicrobiol Rev. 2020; 33(4): e00028-20. doi:10.1128/CMR.00028-20.
- O’Maley JJ, Hartman FW, Treatment of ınfluenzal pneumonia with plasma of convalescent patientsJAMA, 1919; pp34.
- Fung M, Nambiar A, Pandev S, Aldrich JM, Teraoka J, et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl Infect Dis. 2020; e13477. doi: 10.1111/tid.13477.
- European Centre for Disease Prevention and Control, COVID-19 situation update worldwide, as of week 50, 2020.
- Senefeld JW, Klassen SA, Ford SK, et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency. medRxiv 2020; 20224790.
- Jamir I, Lohia P, Pande RK, Setia R, et al. Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumonia. Ann Hepatobiliary Pancreat Surg. 2020; 24(4): 526-532. doi: 10.14701/ahbps.2020.24.4.526.
- London J, Boutboul D, Lacombe K, et al. Severe COVID-19 in Patientswith B Cell Alymphocytosis and Response to Convalescent Plasma Therapy. J ClinImmunol, 2020.
- Baang JH, Smith C, Mirabelli C, Valesano AL, et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient, The Journal of Infectious Diseases, jiaa666.
- Hueso T, Pouderoux C, Péré H, Beaumont AL, et al.Convalescent plasma therapyfor B-cell–depleted patients with protracted COVID-19. Blood 2020; 136 (20): 2290–2295.
- Senefeld JW, Klassen SA, Ford SK, et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency medRxiv 2020; 20224790.
- Moore JL, Ganapathiraju PV, Kurtz CP, Wainscoat B. A 63-Year-Old Woman with a History of Non-Hodgkin Lymphoma with Persistent SARS-CoV-2 Infection Who Was Seronegative and Treated with Convalescent Plasma. The American journal of case reports, 21, e927812.
- Van Damme FAK, Tavernier S, Van Roy N, et al. Case Report: Convalescent Plasma, a Targeted Therapy for Patients with CVID and Severe COVID-19 Front. Immunol., 2020.
- Ibrahim M, Pal P, Niu A, et al. COVID-19 convalescent plasma decreased oxygen requirement and hospital stay in COVID-19 hospitalized patients including those with hematological malignancies: a report of 16 patients. Presented at: the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition; December 5-9, 2020. Abstract 101
- Alsharidah S, Ayed M, Ameen R M, Alhuraish F, et al. 4COVID-19 Convalescent Plasma Treatment of Moderate and Severe Cases of SARS-CoV-2 Infection: A Multicenter Interventional Study. Int J Infect Dis. 2020; S1201-9712(20); 32513-3. doi: 10.1016/j.ijid.2020.11.198.
- Yoon H ah, Bartash R, Gendlina I, Rivera J et al. Treatment of Severe COVID-19 with Convalescent Plasma in the Bronx, NYC. medRxiv 2020.12.02.20242909.
- Libster R, Marc GP, Wappner D, CovielloS, et al. Prevention of severe COVID-19 in the elderly by early high-titer plasma. medRxiv 2020.11.20.20234013.
- Salazar E, Christensen PA,Graviss EA, Nguyen DT, et al. Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG Am J Pathol. 2020; 191(1): 90-107. doi: 10.1016/j.ajpath.2020.10.008.
- Salazar E, Christensen PA,Graviss EA, Nguyen DT, et al. Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality Am J Pathol. 2020; 190(11): 2290-2303. doi: 10.1016/j.ajpath.2020.08.001.
- Ahmad A, Salsabil M, Oliver T. Mortality rates in matched cohort, pseudo-randomised and randomised trials of convalescent plasma given to COVID-19 patients medRxiv 2020.11.19.20234757.
- Clarifying the Emergency Use Authorization Framework For COVID-19 Convalescent Plasma: Considerations for Clinicians Prepared Jointly by The Infectious Diseases Society of America and AABB, 2020.

