Ampullary Mass and Melena: An Unusual Presentation of an Unusual Tumor
Ahmad M Al-Taee1, Danielle Carpenter2 and Jason R Taylor3
1Division of Gastroenterology and Hepatology, Department of Internal Medicine, New York University School of Medicine, USA
2Department of Pathology, Saint Louis University School of Medicine, USA.
3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Saint Louis University School of Medicine, USA
Received Date: 31/07/2021; Published Date: 26/08/2021
*Corresponding author: Ahmad M Al-Taee, MD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, New York University School of Medicine, New York, NY. USA. Address: 3635 Vista Ave, 9th Floor FDT, St Louis, MO 63104
Abstract
Hepatoid Adenocarcinoma (HAC) is a rare extrahepatic adenocarcinoma with histologic and immunohistochemical evidence of hepatocellular differentiation. The vast majority of tumors involve the stomach and small bowel involvement is exceedingly rare. Here we present the case of an 81-year-old male who presented to the emergency room with melena and was found to have an ampullary mass and hepatic lesions. Histologic and immunohistochemical examination of biopsies obtained from the mass revealed HAC. This diagnosis should be considered in patients with an ampullary mass and multiple liver masses. Immunohistochemical stains can help distinguish HAC from other more common diagnoses such as ampullary adenocarcinoma and hepatocellular carcinoma as this can have prognostic and therapeutic implications.
Introduction
Hepatoid Adenocarcinoma (HAC) is a rare extrahepatic adenocarcinoma with histologic and immunohistochemical evidence of hepatocellular differentiation. The vast majority of tumors involve the stomach and small bowel involvement is exceedingly rare. Histologic examination with Immunohistochemical (IHC) staining of tissue biopsies is considered the gold standard for diagnosis. Differentiating HAC from other more common diagnoses is important as this can have prognostic and therapeutic implications.
Case Presentation
An 81-year-old man presented to an emergency room at an outside facility with a 4-day history of melena. Past medical history was notable for deep venous thrombosis for which he was on apixaban. There was no history of use of aspirin or other non-steroidal anti-inflammatory drugs. Vital signs were within normal limits. Physical examination showed a soft abdomen without tenderness or palpable masses. Digital rectal examination showed melenic stools.
Laboratory evaluation showed a hemoglobin of 5.7 g/dL (reference range 12.7-16.8 g/dL), a mean corpuscular volume of 79 fL (reference range 83-11 fL) and a blood urea nitrogen of 24 mg/dL (reference range 5-23 mg/dL). Platelet count, INR and liver enzymes were all within reference range. He received two units of packed red blood cells with appropriate improvement in hemoglobin to 8.8 g/dL. After adequate resuscitation, he underwent an upper endoscopy which revealed a 3-cm mass at the area of the papilla (Figure 1, panel A), likely the source of melena. Abdominal computed tomography with intravenous contrast showed the papillary mass along with upstream biliary and pancreatic ductal dilation and multiple hepatic masses concerning for metastasis.
Patient was then referred to our hospital for evaluation of the ampullary mass. We performed upper endoscopy that showed a 3-cm ulcerating and fungating mass at the area of the papilla (Figure 1, panel B). Biopsies were taken for histologic examination. Endoscopic Ultrasound (EUS) revealed a 23x23 mm heterogenous irregular mass in the area of the ampulla of Vater resulting in distal biliary stricture. The common bile duct was dilated up to 18 mm. There was sonographic evidence of invasion into the serosa and pancreas. EUS also showed several irregular and heterogenous lesions in the right and left hepatic lobes concerning for metastasis. Fine Needle Aspiration (FNA) was not performed due to the concern for worsening biliary obstruction from edema resulting from FNA. Histologic examination of the biopsies obtained from the ampullary mass revealed poorly differentiated adenocarcinoma. Immunohistochemical staining was positive for glypican 3 (Figure 2), Alpha-Fetoprotein (AFP), CDX2, and CK19, but was negative for CK7 and CK20, suggestive of Hepatoid Adenocarcinoma (HAC).
Repeat EUS with FNA of one of the hepatic masses revealed poorly differentiated adenocarcinoma with immunohistochemical staining profile that is essentially similar to the ampullary mass. Therefore, patient was diagnosed with hepatoid adenocarcinoma of the ampulla of Vater with local invasion and hepatic metastasis. Distal biliary obstruction from the ampullary mass was managed with insertion of biliary stents during an endoscopic retrograde cholangiopancreatography. Patient was referred to oncology for further management.
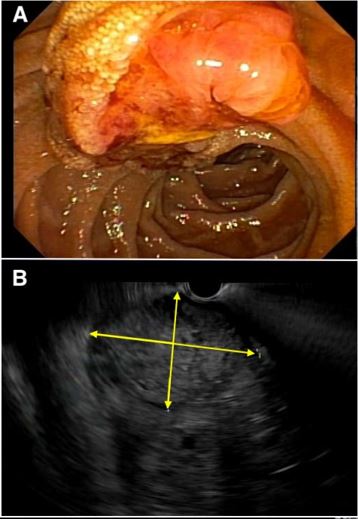
Figure 1: Upper endoscopy showed a 3-cm ampullary mass (A), which was confirmed with endoscopic ultrasound (EUS) (B). The yellow arrows define the extent of the mass on EUS.
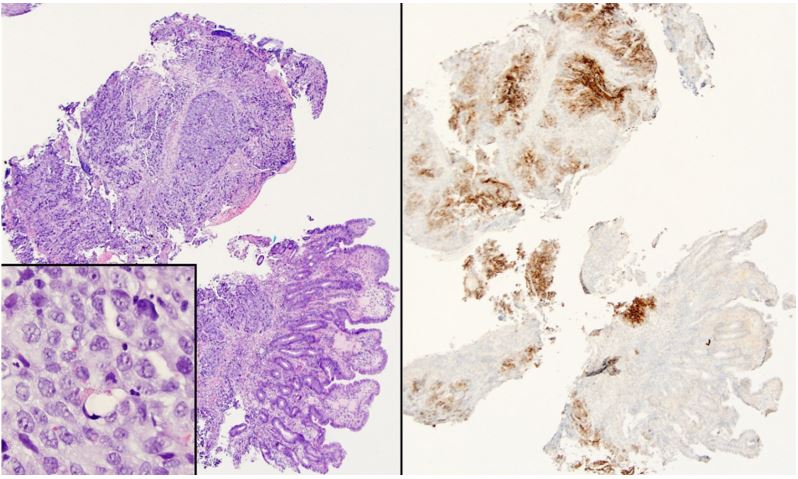
Figure 2: (Left panel) Sheets of tumor cells infiltrate the deep mucosa; higher power (inset) shows abundant cytoplasm and occasional hyaline globules (hematoxylin and eosin). (Right panel) Glypican 3 immunostaining is diffusely positive.
Discussion
HAC is a rare extrahepatic malignancy with histologic and immunohistochemical evidence of hepatocellular differentiation [1]. It most commonly originates in the stomach, but can arise from other organs [2] such as gallbladder [3-5], colon [6,7], lungs [8,9], endometrium [10], orbits [11], urinary bladder [12], peritoneal cavity [13], among others. Involvement of the small bowel is exceedingly rare and only two case reports of ampullary HAC have been published before [14,15]. Serum tumor markers such AFP are elevated in some but not all HACs [16]. Histologic examination with Immunohistochemical (IHC) staining of tissue biopsies is considered the gold standard for diagnosis. The overall prognosis is poor and therefore early diagnosis is crucial [17]. There is no standard treatment regimen for HAC and the therapeutic approach is based on organ of origin.
In our case, the combination of positive IHC staining for hepatocellular markers such as glypican 3, AFP, and CDX2 along with negative staining for CK7 and CK20 is an unusual immunophenotype. The main differential diagnosis is hepatocellular carcinoma (HCC). However, the diffuse staining for CDX2 and CK19 with the ampullary location of the tumor makes HCC unlikely. Moreover, an additional immunostain, SALL4, was obtained and shows strong nuclear staining in tumors cells. SALL4 in an oncofetal protein and positive staining has been reported in poorly differentiated, fetal-type carcinomas of the gastrointestinal tract including HAC [18-20]. These findings are suggestive of HAC originating in the ampulla and resulting in hepatic metastasis. Furthermore, the presence of hepatocellular markers argues against a diagnosis of primary ampullary adenocarcinoma. Distinguishing HAC from primary ampullary adenocarcinoma is important as this may have therapeutic implications. One report has shown lack of sensitivity of such tumors to gemcitabine and 5-fluorouracil [15], two agents used in the management of ampullary adenocarcinoma.
HAC should be considered in patients with an ampullary mass and multiple liver masses. Immunohistochemical stains can help distinguish HAC from other more common diagnoses such as ampullary adenocarcinoma and HCC as this can have prognostic and therapeutic implications.
Conflicts of Interest and Financial Support: The authors report no conflicts of interest or funding.
Authors roles: AMA, DC, and JRT drafted the manuscript. AMA and JRT provided the endoscopic and sonographic images while DC provided the histologic figures. All authors have reviewed and agreed on the final draft prior to submission. This work has not been presented in any professional meeting.
References
- Ogbonna OH, et al. Hepatoid Adenocarcinoma of the Duodenum: An Unusual Location. Case Rep Oncol, 2016; 9(1): p. 182-187.
- Su JS, et al. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol, 2013; 19(3): p. 321-327.
- Devi, N.R., et al., Hepatoid Adenocarcinoma of the Gall Bladder-A Rare Variant. J Clin Diagn Res, 2015; 9(8): p. ED09-ED10.
- Ellouze S, et al. Hepatoid adenocarcinoma of the gallbladder. World J Surg Oncol, 2011; 9: p. 103.
- Gakiopoulou H, et al. Hepatoid adenocarcinoma of the gallbladder. Dig Dis Sci, 2007; 52(12): p. 3358-3362.
- Armaghani A, Hernandez Gonzalo D, Daily K. Hepatoid adenocarcinoma of the colon. BMJ Case Rep, 2015.
- Cappetta A, et al. Hepatoid adenocarcinoma of the colon: what should we target? Pathol Oncol Res, 2012; 18(1): p. 93-96.
- Arnould L, et al. Hepatoid adenocarcinoma of the lung: report of a case of an unusual alpha- fetoprotein-producing lung tumor. Am J Surg Pathol, 1997; 21(9): p. 1113-1118.
- Ayub A, et al. Pulmonary hepatoid adenocarcinoma. J Thorac Cardiovasc Surg,
- Adams SF, et al. An alpha-fetoprotein-producing hepatoid adenocarcinoma of the Gynecol Oncol, 2001; 83(2): p. 418-421.
- Alsberge JB, et al. Primary hepatoid adenocarcinoma of the orbit. Am J Ophthalmol Case Rep, 2017; 5: p. 38-40.
- Burgues O, et al. Hepatoid adenocarcinoma of the urinary bladder. An unusual Virchows Arch, 1999; 435(1): p. 71-75.
- Chen X, et al. Hepatoid adenocarcinoma in the peritoneal cavity: Two case reports. Medicine (Baltimore), 2019; 98(5): p.
- Gardiner GW, G Lajoie, Keith R. Hepatoid adenocarcinoma of the papilla of Histopathology, 1992; 20(6): p. 541-544.
- Takahashi N, et al. Establishment and biological characterization of a novel cell line derived from hepatoid adenocarcinoma originated at the ampulla of Vater. Int J Oncol, 2014; 44(4): p. 1139-1145.
- Baek SK, et al. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol, 2011; 11: p.
- Zeng X, et al. Clinicopathological features and prognosis of intestinal hepatoid adenocarcinoma: evaluation of a pooled case series. Oncotarget, 2018; 9(2): p. 2715-2725.
- Miettinen M, et al. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol, 2014; 38(3): p. 410-420.
- Kwon MJ, et al. Gastric adenocarcinoma with enteroblastic differentiation should be differentiated from hepatoid adenocarcinoma: A study with emphasis on clear cells and clinicopathologic spectrum. Pathol Res Pract, 2019: p.
- Osada M, et al. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol, 2014; 45(6): p. 1243-1250.

