“Stable” Asystole in a Patient with Left Ventricular Assist Device
Nino Nozadze1,*, Raveen Chawla2, Mark Schoenfeld4, Steven Zweibel3 and Jason Gluck2
1Department of Medicine, University of Connecticut
2Advanced Heart failure and Transplant Cardiology, University of Connecticut, Hartford Hospital
3Department of Interventional Electrophysiology, Hartford Hospital
4Electrophysiology, Yale University School of Medicine, New Haven, Connecticut
Received Date: 24/07/2021; Published Date: 18/08/2021
*Corresponding author: Nino Nozadze, Department of Medicine, University of Connecticut, USA
Background
Left Ventricular Assist Devices (LVADs) for mechanical circulatory support are rapidly increasing with more than 25,000 LVADs implanted over the past decade [1]. Indications for use are patients with end-stage heart failure as a bridge-to-transplant, bridge-to-decision or as Destination Therapy (DT) for those not deemed transplant candidates [2,3]. LVAD therapy changed the landscape of advanced heart failure management; however, they are not without complications such as gastrointestinal bleeding, pump thrombosis, stroke, infection and right heart failure. One area of interest is arrhythmia in the LVAD supported patient. Although presentation and consequences of Ventricular Arrhythmias (VA) in LVAD supported patients are well described and often stabilized by the mechanical support, presence of asystole in LVAD patients is less commonly reported.
We describe a case of a patient with HeartMate II LVAD presenting with orthostasis and low flow alarms and incidentally noted to be in “stable” asystole, remaining conversant and relatively well perfused at rest and ambulation. This case describes unique challenges that LVADs can pose to clinicians and highlights an area of improvement of provider education in this rapidly growing specialized patient subgroup.
Case Presentation
Our patient is a 75-year-old male with coronary artery disease status post coronary artery bypass surgery complicated by advanced ischemic cardiomyopathy requiring cardiac resynchronization therapy-defibrillator (CRT-D) and ultimately HeartMate II LVAD as destination therapy. Post implantation, his CRT-D was abandoned after it reached the elective replacement indicator as he had no ventricular arrhythmia and was not pacer dependent.
He presented to the ED via EMS for new onset of low flow alarms that started earlier in the day. On presentation he was stable, pleasant and in a humorous mood. He reported feeling lightheaded when standing up but denied any syncope. He also denied worsening dyspnea, orthopnea, paroxysmal nocturnal dyspnea, or lower extremity edema. While in the ED, the patient was noted to be asystolic (Figure 1). His mean arterial pressure was in the 50s-60s. Physical exam was unremarkable with the expected LVAD hum and lack of pulses. LVAD parameters on interrogation revealed RPM 9400, Flow 2.5 (baseline 5.1), Pulsatility Index (PI) 2.7, Power 4.4 (baseline 5.1). Patient was evaluated by advanced heart failure service and electrophysiology in the ED and subsequently underwent implantation of a new battery with reactivation of his previously abandoned pacemaker leads for right ventricular (RV) pacing with resultant hemodynamic improvement. Patient’s LVAD parameters after pacemaker reactivation were Flow 4.1, Pulsatility Index 4.9 and power of 5.3. Ultimately, he was discharged home in stable condition.
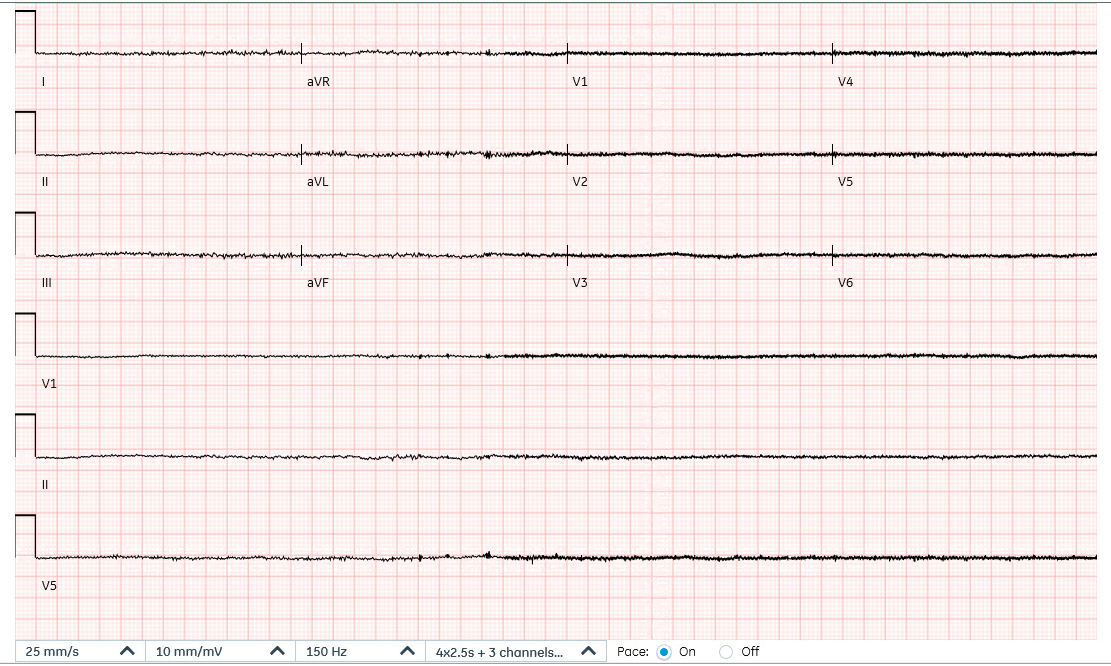
Figure 1: ECG demonstrating asystole with baseline interference due to background left ventricular assist device activity.
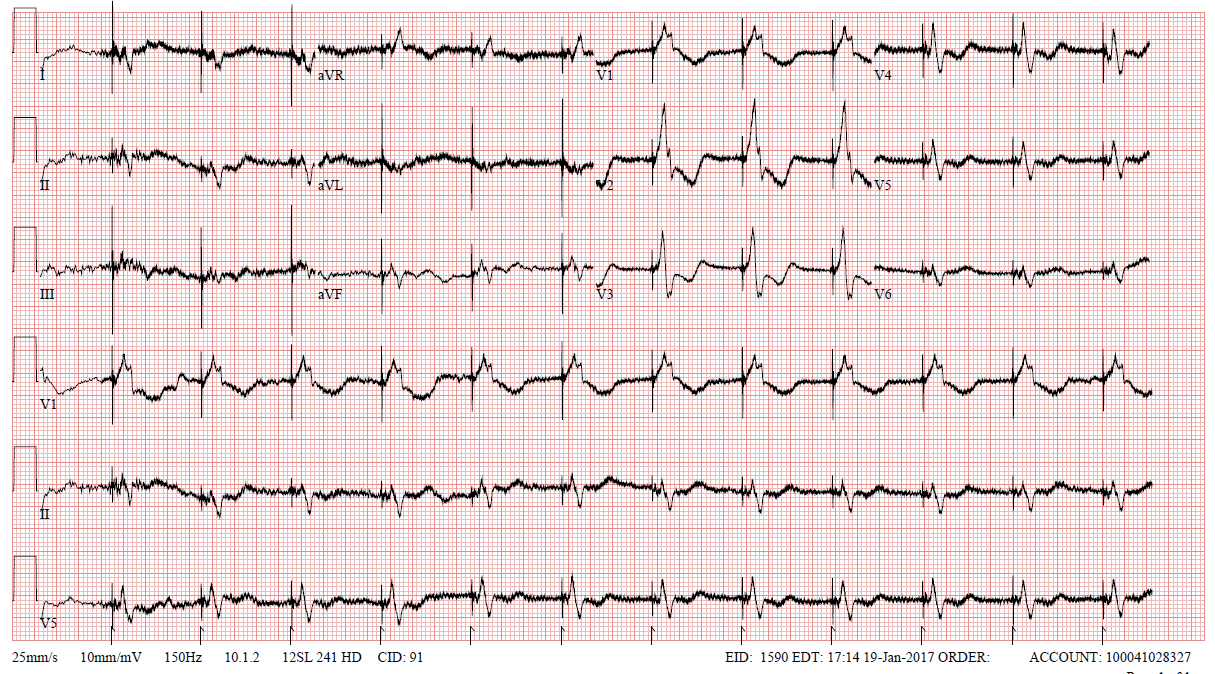
Figure 2: Baseline ECG with LVAD support and CRT-D prior to abandonment.
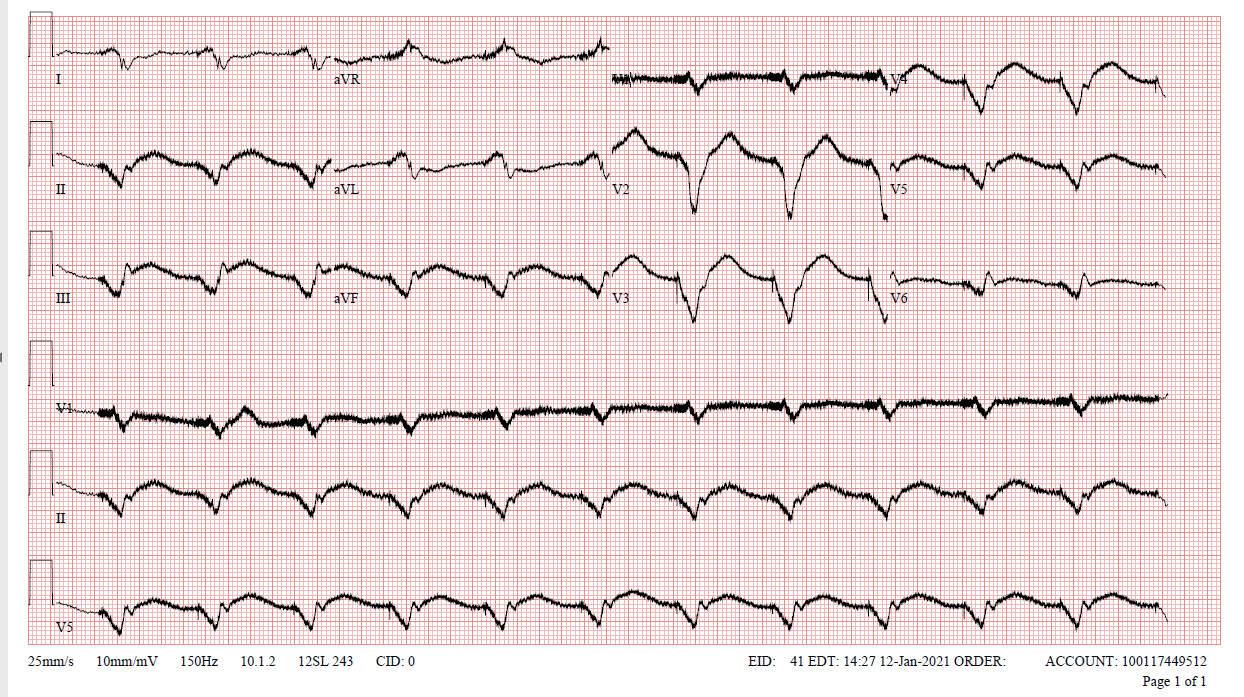
Figure 3: ECG after pacemaker reactivation.
Discussion
Continuous-flow LVADs continuously propel blood from the left ventricle to the aorta at varying rates. They are preload dependent and therefore often dependent on Right Ventricular (RV) function. Conditions that reduce preload such as ventricular arrhythmias, right heart failure and asystole can cause decreased RV filling and reduce the flow through the LVAD; however, in some cases, patients can develop a quasi-Fontan circulation with blood being “pulled” through the RV and pulmonary bed resulting in fairly well tolerated ventricular arrhythmia and even asystole.
Patients can remain hemodynamically stable in these conditions with minimal symptoms, however, most will eventually decompensate due to inadequate LVAD flow, although that time period is not well defined [4,5]. Very few cases of asystolic LVAD patients without immediate hemodynamic collapse are described. Similarly, to with ventricular arrhythmias, the hypothesized physiology for stability is this quasi-Fontan circulation, where low pulmonary vascular resistance with elevated central venous pressure allows pulmonary vasculature perfusion and left atrial filling [4,6,7].
Hemodynamic parameters displayed by the LVAD are RPM (programmed pump speed), Flow, Pulsatility Index and Power. Flow is calculated from pump power consumption and is not necessarily an equivalent to actual flow through the LVAD. When preload and afterload are constant, pump flow is directly proportional to the programmed pump speed. Low preload conditions such as hypovolemia and inflow obstruction (which mimics low preload) cause low flow. In the case of a pump outflow obstruction, blood flow slows as resistance rises and in the absence of flow, power consumption decreases. Thus, the drop in displayed pump flow can be due to low pump speed, reduced preload or increased afterload [4].
In the case of our patient, low flow alarms and decreased PI were due to decreased LV preload in the setting of asystole. The conventional differential diagnosis to keep in mind with low flow and low PI are volume depletion, thrombotic occlusion at the inflow or outflow sites of the VAD, right heart failure and ventricular arrhythmias [4]. Asystole should also be considered among differential diagnosis.
Ventricular arrhythmias remain common after LVAD implantation, asystole, as in the present case can equally be an issue. This is particularly true as many of LVAD supported patients have CRT-Ds implanted in the setting of pre-existent left bundle branch blocks, which can progress to complete heart block or asystole even if the patient was not previously pacer dependent. Asystole in our patient likely occurred due to progression of his conduction system disease.
An important issue being emphasized in the current case is the role of ICDs and pacemakers in LVAD supported patients, which remains somewhat nebulous due to lack of randomized clinical trial data. As this case demonstrates, LVAD supported patients can derive benefit from pacing/generator replacement; however, if not done, the LVAD can stabilize an otherwise lethal arrhythmia and allow patients to seek medical care when a pacemaker/ICD may be needed.
Conclusion and Future Perspective
Traditionally unstable asystole and ventricular arrhythmia can be well tolerated in the LVAD population due to quasi-Fontan physiology. As the LVAD population expands, so too must the education of providers on the potentially stabilized presentations of both ventricular arrhythmia and traditionally lethal arrhythmia such as asystole in the LVAD population among all healthcare providers. The role of ICD/Pacemaker in post LVAD patients remains nebulous; however, when these patient’s present with rhythmic “impossibilities” it behooves providers to consider them more likely LVAD stabilized, and not LVAD interference. Yes, there may be such a thing as, “stable” asystole.
References
- Teuteberg JJ, Cleveland JC Jr, Cowger J, Higgins RS, Goldstein DJ, Keebler M, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg. 2020; 109(3): 649-660. doi: 10.1016/j.athoracsur.2019.12.005. PMID: 32115073.
- Miller L, Birks E, Guglin M, Lamba H, Frazier OH. Use of Ventricular Assist Devices and Heart Transplantation for Advanced Heart Failure. Circ Res. 2019; 124(11): 1658-1678. doi: 10.1161/CIRCRESAHA.119.313574. PMID: 31120817.
- AATS/ISHLT guidelines on mechanical circulatory support.
- Lim HS, Howell N, Ranasinghe A. The Physiology of Continuous-Flow Left Ventricular Assist Devices. J Card Fail. 2017; 23(2): 169-180. doi: 10.1016/j.cardfail.2016.10.015. Epub 2016 Oct 29. PMID: 27989869.
- Gopinathannair R, Cornwell WK, Dukes JW, Ellis CR, Hickey KT, Joglar JA, et al. Device Therapy and Arrhythmia Management in Left Ventricular Assist Device Recipients: A Scientific Statement from the American Heart Association. Circulation. 2019 May 14;139(20):e967-e989. doi: 10.1161/CIR.0000000000000673. PMID: 30943783.
- Imamura T, Kinugawa K, Nitta D, Kinoshita O, Nawata K, Ono M. Fontan-Like Hemodynamics Complicated with Ventricular Fibrillation During Left Ventricular Assist Device Support. Int Heart J. 2016; 57(4): 515-51 doi: 10.1536/ihj.16-008. Epub 2016 Jul 7. PMID: 27385606.
- Javed W, Chaggar PS, Venkateswaran R, Shaw SM. Prolonged asystole in a patient with an isolated left ventricular assist device. Future Cardiol. 2016; 12(5): 533-53 doi: 10.2217/fca-2016-0022. Epub 2016 Aug 19. PMID: 27539188.

