Hodgkin Lymphoma with Ophelia Syndrome: When Paraneoplastic Syndrome as the Only Manifestation of Hodgkin Lymphoma
Yeow Hoay Koh*
Duke-NUS Medical School, National Neuroscience Institute of Singapore, Singapore
Received Date: 30/07/2021; Published Date: 17/08/2021
*Corresponding author: Yeow Hoay Koh, Duke-NUS Medical School, National Neuroscience Institute of Singapore, Singapore
Abstract
Background: Limbic encephalitis is a neurological syndrome characterized by inflammation of the brain with alterations in behavior and cognition. Inflammation of the medial temporal lobes on brain imaging is most characteristic. The association between limbic encephalitis and Hodgkin’s lymphoma has been given the eponym of Ophelia syndrome. Ophelia syndrome is a rare paraneoplastic neurological syndrome (PNS). Although the medial temporal lobe of the brain is commonly involved in Ophelia syndrome, other brain regions may also be involved. Here, we report an unusual case of Ophelia syndrome: a 44-year-old man who presented with limbic encephalitis, and subsequently diagnosed to have Hodgkin lymphoma). In addition to the usual bilateral mesial temporal lobes involvement, the MRI brain also showed unihemispheric cerebral involvement and gyral swelling, which was not reported in the literature so far.
Keywords: Ophelia syndrome; Limbic encephalitis; Hodgkin lymphoma; MRI; Paraneoplastic neurological syndrome
Introduction
The paraneoplastic syndrome can involve diverse areas of the peripheral nervous system and central nervous syndrome (CNS), including the muscles, neuromuscular junction, peripheral nerve, autonomic system, and other distinct brain regions [1]. This rare disorder arises from immune cross-reactivity between malignant and normal tissues or tumor secretion of hormones, antibodies, or cytokines. Paraneoplastic Neurological Syndrome (PNS) usually results from tumor-directed antibodies, so-called onconeural antibodies, and associated antigen-specific T-lymphocytes, which attack the central or peripheral nervous system [2]. Paraneoplastic neurological syndromes are rare complications of Hodgkin lymphoma [3]. The PNS subtypes that commonly seen in Hodgkin lymphoma are cerebellar degeneration and limbic encephalitis [2,7] (also known as Ophelia syndrome). Here, we report an unusual case of Ophelia syndrome with involvement of bilateral mesial temporal and the left cerebral hemisphere, which later diagnosed to have Hodgkin lymphoma.
Case Study
A 44-year-old man presented with headache, progressive reduction of short-term memory, mood change, and intermittent speech difficulties for one month. He was subsequently admitted for generalized tonic-clonic seizure. There was no fever, weight loss, sweating, and a history of trauma. On admission, he was treated as status epilepticus as he had a total of 6 episodes of clinical seizures with no regain of consciousness in between the episodes. Limited physical examination on admission showed regular pupil sizes with no papilloedema. There was no fever and no neck stiffness. He was found to have brisk right knee, biceps and ankle reflexes. The extensor plantar response was positive over the right.
The electroencephalography (EEG) showed epileptiform activities lateralized to the left hemisphere [Figure1]. MRI brain revealed cortical gyral swelling of bilateral medial temporal lobe, unilateral left cerebral hemisphere, and left insular ribbon [Figure 2, 3]. The MR angiogram was normal, and MR venogram did not show cerebral venous thrombosis. Cerebrospinal Fluid (CSF) analysis showed slight pleocytosis (white blood cells 6/μL), mildly raised CSF protein 0.44 g/L (normal range <0.40g/L). CSF cultures, Gram stain, TB PCR, mumps and measles antibody, HSV PCR testing, and anti-NMDA receptor antibodies were negative. His blood count, renal, thyroid and liver function tests, serum electrolytes, HIV serology, Hepatitis B and C screening, VDRL, ESR, pro-calcitonin, C-reactive protein, HbA1c were unremarkable. Tumor markers (CA19-9, alpha-fetoprotein, CEA, and PSA) were normal. Antinuclear Antibody (ANA) and Extractable Nuclear Antigen (ENA) antibodies panel were negative.
His Chest X-Ray (CXR), CT thorax, abdomen, and pelvis were unremarkable. Further investigation for anti-NMDA receptor, anti-Tr, ANNA, PCA2, Ma1/2, CRMP5, amphiphysin, Glutamic Acid Decarboxylase (GAD), GABA-B receptor, SOX-1, LGI, and CASPR2 were all negative.
He was treated as autoimmune encephalitis with two courses of intravenous immunoglobulin and two courses of pulse intravenous methylprednisolone 1gm daily for five days, followed by a tapering dose of oral steroid. His seizure was well controlled with two types of anti-epileptics agents (Levetiracetam and sodium valproate). Two months from the admission, the neurological symptoms have nearly resolved. Repeat MRI brain showed near-complete resolution of the left hemisphere and both mesial temporal lobe swelling [Figure 4]. The repeat EEG was also normal. However, the etiology of the autoimmune encephalitis was still uncertain. A Fluorodeoxyglucose PET/CT study was performed, and it showed an intensely hypermetabolic lesion in the anterior mediastinum [Figure 5]. Moderately hypermetabolic borderline right supraclavicular lymph node and mildly hypermetabolic mediastinal nodes were seen.
He underwent thymectomy, and the thymic mass pathology showed nodular sclerosis type Hodgkin’s disease. He was treated with ABVD chemotherapy, followed by radiotherapy by his oncologist. His oral steroid and anti-epileptics were discontinued after one year. Two years from the initial presentation, his neurological deficits, including his cognitive function, have all returned to the baseline.
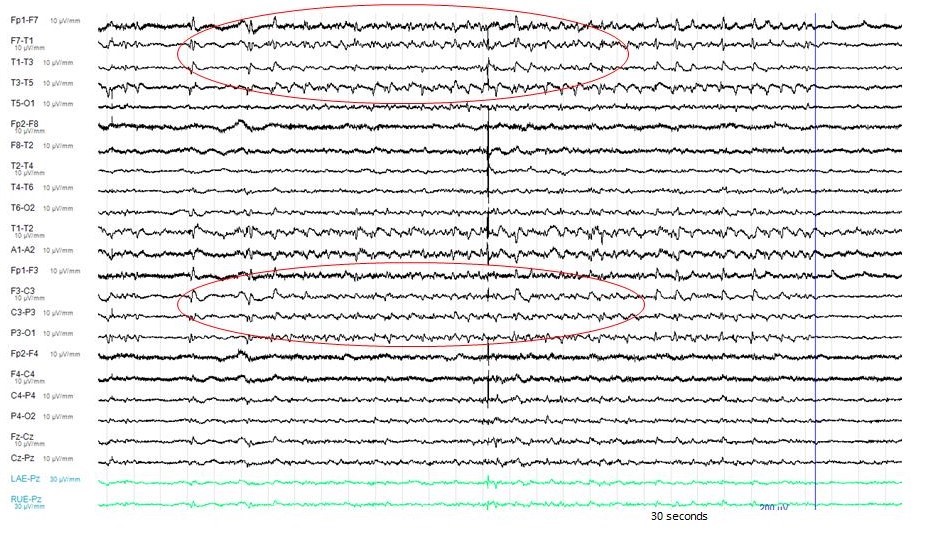
Figure 1: The Electro-Encephalography (EEG) showed epileptiform activities lateralized to the left hemisphere.
Figure 2: The MRI brain (axial view) showed T2 hyperintense signal over bilateral medial temporal lobe, unilateral cortical gyrus of the left cerebral hemisphere and left insular ribbon.
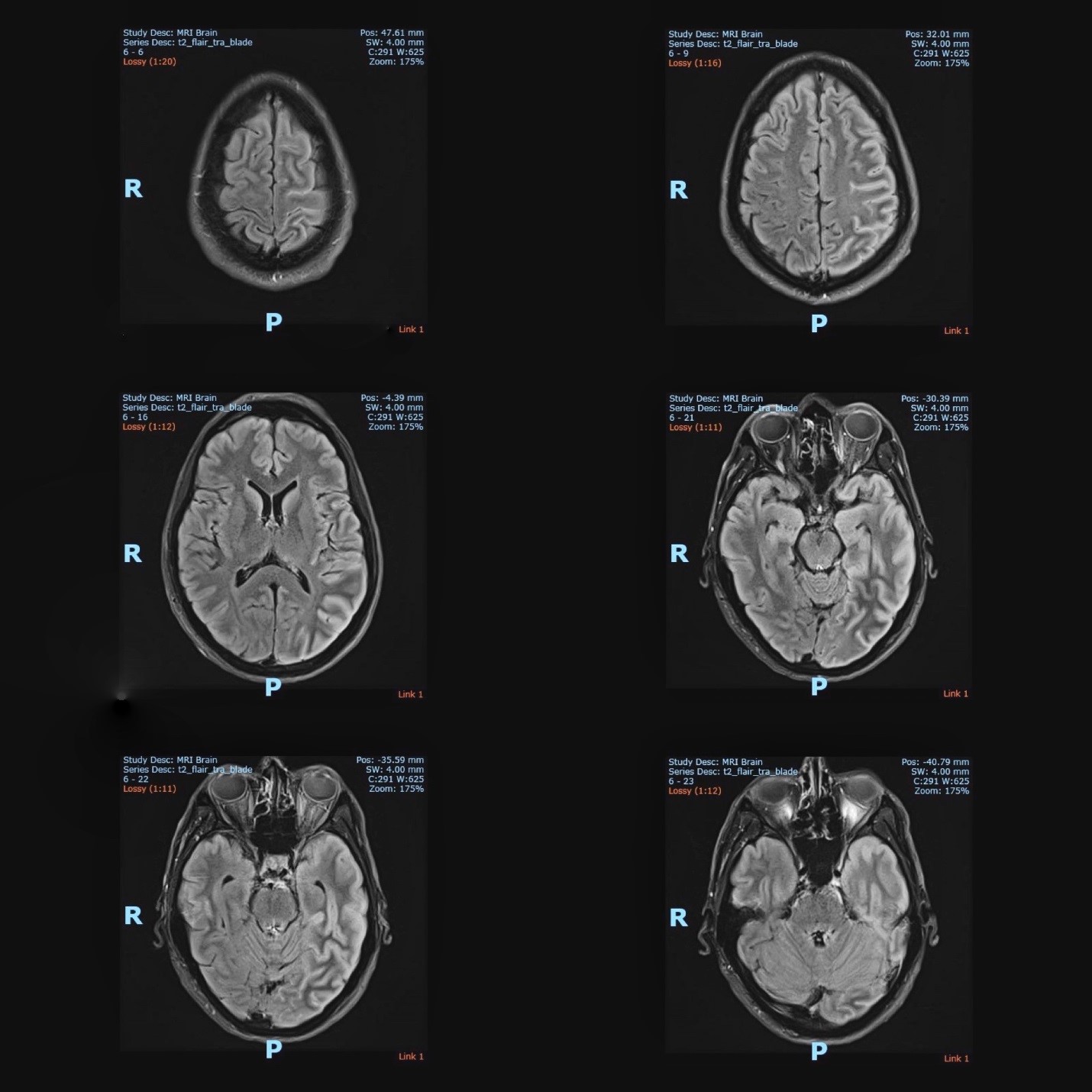
Figure 3: The MRI brain (coronal view) showed T2 hyperintense signal over bilateral medial temporal lobe, unilateral cortical gyrus of the left cerebral hemisphere and left insular ribbon.
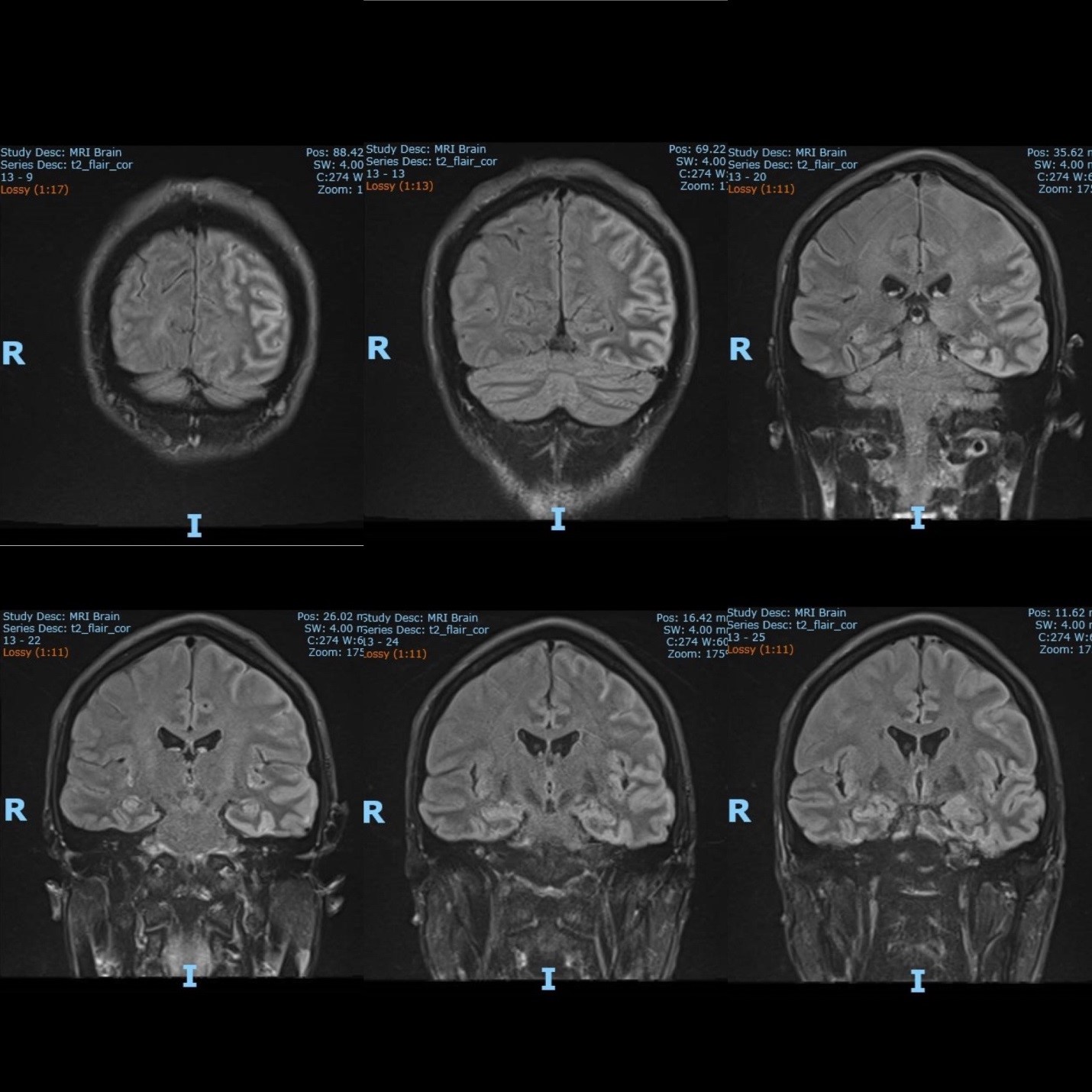
Figure 4: Repeat MRI brain (2 months later) showed near complete resolution of the left hemisphere and both mesial temporal lobes swelling.
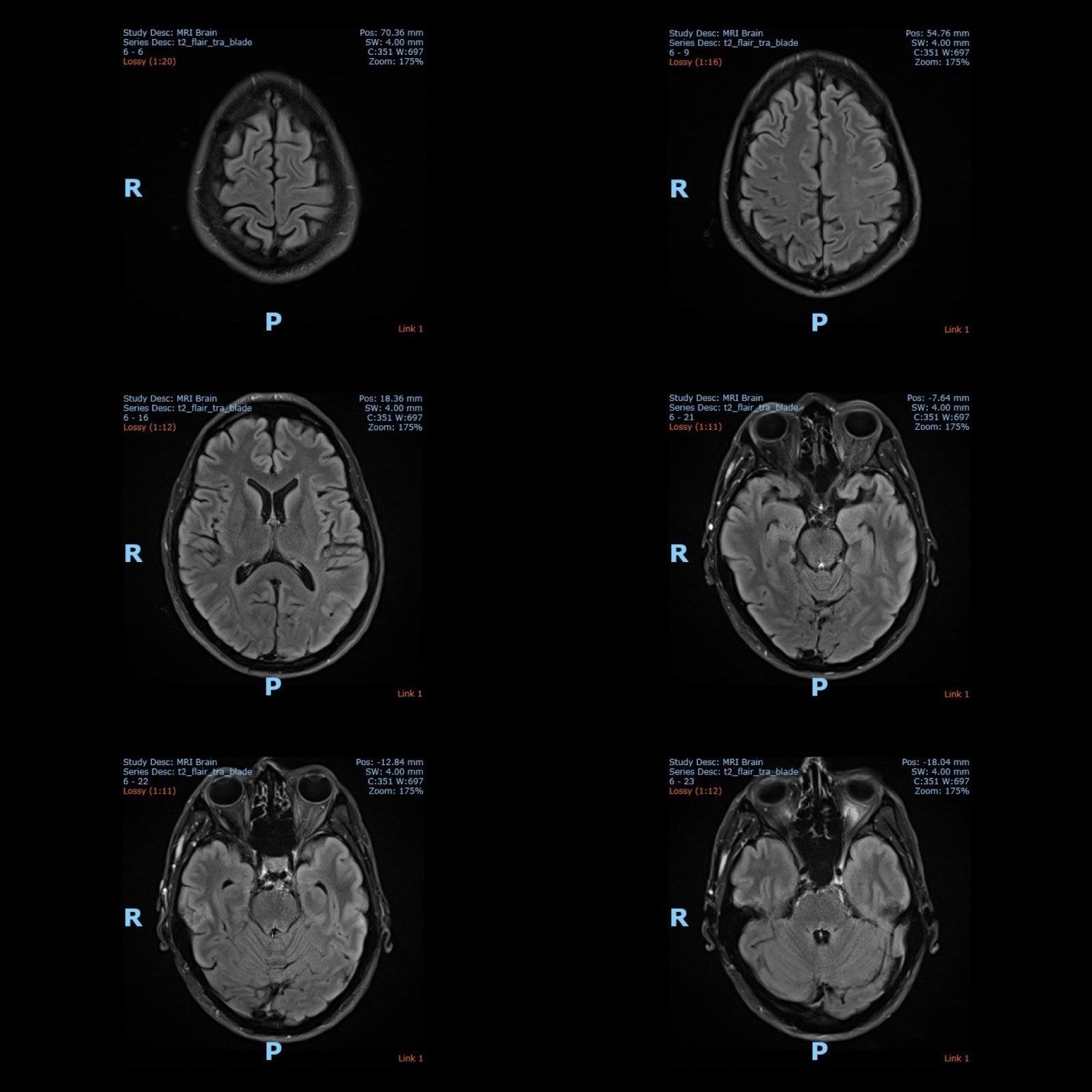
Figure 5: Fluorodeoxyglucose PET/CT Study was performed and it showed intensely hypermetabolic lesion in the anterior mediastinum
Discussion
Limbic encephalitis is a neurological syndrome characterized by inflammation of the brain with alterations in behavior and cognition, inflammation of the medial temporal lobes on brain imaging is most characteristic, but other brain regions may also be involved [1]. Most of the CNS autoantibodies can result in some form of limbic encephalitis [8]. Limbic encephalitis can be broadly categorized into two groups: the first group which is associated with antibodies against intracellular neuronal antigens (frequently associated with cancers and responds poorly to treatment), and the second group with antibodies directed against cell membrane or extracellular antigens (less frequently associated with cancers, and responds favorably to immunotherapy) [9, 10].
Limbic encephalitis is one of the neurological emergencies, and treatment needs to be commenced early to reduce long term morbidity and mortality [7]. However, full neuronal-specific antibody testing results can take several weeks, and testing for some of the newest antigens may not be available in most of the centres. As seen in this case, it is challenging for treating Neurologists to treat a critically ill patient for a prolonged period with incomplete information. The recent advance to the diagnostic criteria for possible autoimmune encephalitis and consensus on beginning empiric immunotherapy for autoimmune encephalitis [11] has facilitated early diagnosis and early immunotherapy in limbic encephalitis.
Another main challenge, in this case, is the initial antibodies testing and screening for malignancy (whole-body CT scan and tumor markers) were all negative. Nevertheless, the subacute clinical presentations of short-term memory decline, mood change, and seizures are in keeping with the diagnosis of limbic encephalitis. The intermittent episodes of speech difficulty could be due to seizure, or the Broca’s area involvement of the encephalitis. The fact that this patient responded well to the immunotherapy again supports the diagnosis of autoimmune or paraneoplastic limbic encephalitis. Abnormal PET scan and biopsy of the thymus have eventually confirmed the etiology of the limbic encephalitis.
A broad differential diagnosis and an extensive workup for autoimmune, paraneoplastic, and infective cause are crucial in patients with limbic encephalitis. The main differential diagnosis for limbic encephalitis is Herpes simplex encephalitis. Diagnosis of Herpes simplex encephalitis was ruled out by negative HSV-PCR in the CSF analysis in this case.
The diagnostic criteria of paraneoplastic limbic encephalitis proposed by Paraneoplastic Neurological Syndromes Euronetwork [12] include subacute onset of seizures, short-term memory loss, confusion and psychiatric symptoms; neuropathological or neuroradiological evidence of limbic system involvement; exclusion of other etiologies of limbic dysfunction; and demonstration of a cancer within 5 years of the diagnosis of the neurological disorder or demonstration of a well-characterized paraneoplastic antibody. Our patient fulfilled the Euronetwork diagnostic criteria for paraneoplastic limbic encephalitis.
The association between limbic encephalitis and Hodgkin’s lymphoma has been given the eponym of Ophelia syndrome [2], in memory of Shakespeare’s character in ‘The Tragedy of Hamlet, Prince of Denmark’. Ophelia syndrome is rare in patient with Hodgkin lymphoma. Although medial temporal lobes involvement is most characteristic in Ophelia syndrome, other brain regions may also be involved.
From our knowledge, unihemispheric cerebral involvement has not been reported among cases of PNS in Hodgkin lymphoma. Unihemispheric cerebral involvement can be seen in a rare chronic neurological disorder, called Rasmussen’s encephalitis. Rasmussen’s encephalitis is characterized by pharmaco-resistant epilepsy, and progressive neurological and cognitive deterioration due to unilateral inflammation of the cerebral cortex [13]. Although Rasmussen encephalitis is classically described as a childhood encephalopathy, the unusual adult-form variant has been described. The adult onset Rasmussen’s syndrome usually exhibit a slower course and a better outcome [13,14]. Nevertheless, this case is clinically not in keeping with Rasmussen’s syndrome.
About half of all autoimmune encephalitis cases are antibody-negative. The detection of auto-antibodies plays an important role in diagnosis and classification of the PNS syndrome. SOX1 antibody, PCA2 antibody, and metabotropic glutamate receptor-5 antibody (mGluR5 Abs) have reported in cases of Ophelia syndrome [15,16]. Knowledge about auto-antibody in Ophelia syndrome is still limited as Ophelia syndrome itself is a rare disorder. In this case, all the antibody tests were negative, including SOX1 antibody and PCA2 antibody. Tests for mGluR5 Abs is not available in the laboratory here.
We conclude that in all cases of limbic encephalitis, or whenever a paraneoplastic disorder is suspected, an extensive tumor workup (including a PET scan) should be initiated promptly. Although paraneoplastic neurological syndrome in Hodgkin lymphoma commonly affecting the mesial temporal regions (Ophelia syndrome), other brain regions may also be involved.
Conflicts of Interest
None.
Funding
This study did not receive any specific grant from funding agencies.
Acknowledgment
None.
References
- Haberlandt E, Bast T, Ebner A, Holthausen H, Kluger G, Kravljanac R, et al. Limbic encephalitis in children and adolescents. Arch Dis Child. 2011; 96: 186-191.
- Carr I. The Ophelia syndrome: memory loss in Hodgkin’s disease. Lancet 1982; 1: 844-845.
- Graus F, Arin H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014; 123: 3230-3238.
- Grimm S, Chamberlain M. Hodgkin’s lymphoma: A review of neurologic complications. Adv Hematol. 2011; 2011: 624578.
- Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 2010; 85: 838e54.
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003; 349: 1543e54.
- Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms,immunological findings and tumour association in 50 patients. Brain 2000; 123: 1481e94.
- Tuzun E, Dalmau J. Limbic encephalitis and variants: Classification, diagnosis and treatment. Neurologist. 2007; 13: 261-271.
- Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain.2005; 128: 1764-1777.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011; 10: 759-772.
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15(4): 391-401. Doi:10.1016/S1474-4422(15)00401-9.
- Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004; 75: 1135-1140.
- Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, et al. Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol 2014; 13: 195-205.
- Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, Kurthen M, et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 2005; 128: 454-471.
- E Lancaster et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011; 77: 1698-1701.
- M Kunstreich et al. Paraneoplastic limbic encephalitis with SOX1 and PCA2 antibodies and relapsing neurological symptoms in an adolescent with Hodgkin lymphoma. Eur J Paediatr Neurol. 2017; 21(4): 661-665. Doi:10.1016/j.ejpn.2017.03.005.

