Candida Auris Bloodstream Infection and Ventriculitis
Kayla R Stover1,*, Himmat S Brar2,3, Risa Webb4 and Maria X Bueno Rios5
1Department of Pharmacy Practice, University of Mississippi School of Pharmacy and University of Mississippi Medical Center, USA
2Department of Medicine, University of Mississippi Medical Center, USA
3Department of Pharmacy Practice, University of Mississippi School of Pharmacy, USA
4Department of Medicine-Infectious Diseases, University of Mississippi Medical Center, USA
5Department of Medicine-Infectious Diseases, University of Mississippi Medical Center, USA
Received Date: 26/07/2021; Published Date: 16/08/2021
*Corresponding author: Kayla R Stover, Department of Pharmacy Practice, University of Mississippi School of Pharmacy and University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216
Abstract
Candida auris infections are associated with high rates of mortality and resistance. Echinocandins are recommended for bloodstream infections, but little information is available for other sources. We present a case of a patient with intracranial hemorrhage who subsequently developed bloodstream infection and ventriculitis with Staphylococcus epidermidis and Candida auris. This presented clinical challenges in treating her infections due to delayed susceptibility testing and concerns with drug penetration to the sites of infection.
Keywords: Invasive Fungal Infection; Echinocandin; Resistance; Infectious Diseases
Introduction
Candida auris infections have been spreading with increasing prevalence since 2015, with inpatient mortality ranging from 30-60% [1]. Outbreaks have been reported, likely because biofilm formation results in invasive infections and colonization of patients, devices, and surfaces, with ability to persist for months [2-3]. Because C. auris may be misidentified in laboratory settings using standard identification methods [4], identification and susceptibility testing can be challenging.
The Centers for Disease Control and Prevention (CDC) reports that 90% of C. auris isolates are resistant to at least one antifungal, and 30% are resistant to at least two antifungals.1 In the United States, 90%, 30%, and less than 5% of isolates have been resistant to fluconazole, amphotericin B, and echinocandins, respectively. Currently, echinocandins are recommended as the treatment of choice for bloodstream infections [5,6].
Case Report
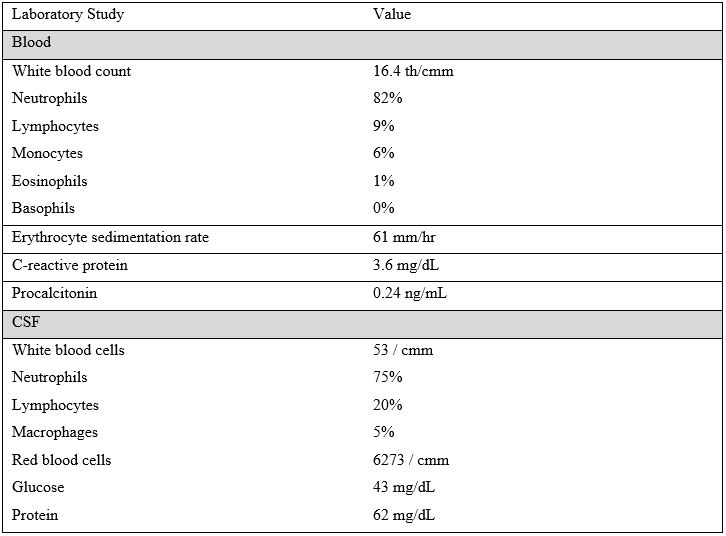
A 64-year-old female with history of hypertension presented after experiencing an intracranial hemorrhage complicated by Cerebrospinal Fluid (CSF) leakage in Africa. There, she received vancomycin and amikacin for sepsis of unknown origin. Physical exam on presentation was significant for a Glasgow Coma Scale of 8 with aphasia and right sided hemiparesis. CT Head revealed ventriculomegaly, left sided hypoattenuation suggestive of edema, and herniation through craniectomy defect with midline shift. The patient underwent craniotomy with external ventricular drain placement. Laboratory studies are in Table 1.
Table 1: Laboratory Studies.
She was started empirically on vancomycin, ceftriaxone, and metronidazole. Gram stain of CSF bacterial cultures
drawn on hospital day 2 demonstrated gram positive cocci. Serology was negative for Cryptococcus, Hepatitis C, HIV, cytomegalovirus, enterovirus, N.meningitidis, Listeria and VDRL was nonreactive. Antimicrobial therapy was escalated to vancomycin and meropenem on day 3 for treatment of ventriculitis. On day 4, her CSF culture grew Staphylococcus epidermidis, and meropenem was discontinued. Micafungin 100 mg daily was added after admission blood cultures resulted positive for yeast. On day 5, the yeast was identified as Candida auris, and the patient was placed on strict contact isolation per hospital protocol. On day 12, CSF gram stain showed yeast. Micafungin was discontinued and the patient was started on amphotericin B and flucytosine. On day 15, the patient developed a spontaneous bilateral parenchymal hemorrhage and was intubated. On day 18, susceptibilities demonstrated resistance to fluconazole. Despite initial improvements, her condition worsened during the hospital stay. The family opted for palliative care, and the patient expired shortly after a compassionate extubation on day 27.
Discussion
This complicated patient was likely infected with C. auris in Africa, possibly from healthcare exposure. Although the bloodstream infection was initially treated appropriately per CDC guidelines [6], little information is available about treatment of C. auris infections of other sites. In one literature review, authors recommend amphotericin B plus flucytosine for infections of the urinary tract or central nervous system (CNS) due to poor penetration of the echinocandins to these sites [7].
Although several C. auris reports have been published, few discuss alternative therapies or CNS infections. In one case series, authors describe 9 patients with fungemia caused by C. auris [8]. All patients were treated with micafungin; two were switched to liposomal amphotericin B after failure to respond to initial therapy. In-hospital mortality was 22%, and 43% of remaining patients were discharged to palliative care. In a case report from 2018, authors describe a patient with nosocomial CSF shunt infection due to multi-drug resistant C. auris [9]. The patient was initially treated with amphotericin and flucytosine. However, this was changed to caspofungin and flucytosine when susceptibilities demonstrated resistance to fluconazole and amphotericin. As a result of persistently positive CSF cultures, intraventricular caspofungin and oral voriconazole were added to the regimen. CSF cultures cleared after 5 days of therapy. Oral voriconazole was continued for 6 weeks, and the patient returned to full activities of daily living by 6 months post-therapy. In another case report, authors describe the course of a patient who developed C. auris fungemia after placement of a ventriculoperitoneal shunt and PICC line insertion [10]. The patient’s CSF culture grew Pseudomonas aeruginosa and blood cultures grew C. auris. The patient was started on micafungin, but amphotericin was added after documentation of persistent fever. After 14 days of therapy, the patient was discharged to a nursing home for further recovery.
In our patient, amphotericin B lipid complex and flucytosine were empirically started due to the expected inadequate penetration of micafungin into the CSF. Unfortunately, the delay in yeast identification, lack of timely susceptibility testing, and clinical complications experienced by the patient confound the picture and make it difficult to determine whether starting this combination earlier would have changed the outcome.
Candida auris is an invasive, multi-drug resistant yeast that has been documented across the world as an emerging pathogen of concern. Early diagnosis, treatment, and isolation is essential for management of these cases and prevention of further transmission.
Conflicts of Interest
Authors have no conflicts of interest to disclose.
Funding Statement
This research did not receive specific funding but was performed as part of the employment of the authors (KRS, CM = University of Mississippi School of Pharmacy; HSB, RW, MXBR = University of Mississippi Medical Center). The employers were not involved in the manuscript writing, editing, approval, or decision to publish.
References
- Candida auris. Centers for Disease Control and Prevention. 2020.
- Hata DJ, Humphries R, Lockhart SR. Candida auris: An Emerging Yeast Pathogen Posing Distinct Challenges for Laboratory Diagnostics, Treatment, and Infection Prevention. Arch Pathol Lab Med. 2020; 144 (1): 107-114.
- Sekyere JO. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. MicrobiologyOpen. 2018; 7(4): e00578.
- Bidaud A, Chowdhary A, Dannaoui E. Candida auris: An emerging drug resistant yeast – A mini-review. Journal de Mycologie Médicale. 2018; 28(3): 568-573.
- Cândido EDS, Affonseca F, Cardoso MH, Franco OL. Echinocandins as Biotechnological Tools for Treating Candida auris Infections. Journal od Fungi. 2020; 6(3): 185.
- Candida auris: information for laboratorians and health professionals. Centers for Disease Control and Prevention. 2019.
- Jeffery-Smith A, Taori SK, Schelenz S, et al. Candida auris: a review of the literature. Clin Microbiol Rev. 2018; 31: e00029-17.
- Park JY, Bradley N, Brooks S, Burney S, Wassner C. Management of Patients with Candida auris Fungemia at Community Hospital, Brooklyn, New York, USA, 2016–2018. Emerging Infectious Diseases. 2019; 25(3): 601–602.
- Singhal T, Kumar A, Borade P, Shah S, Soman R. Successful treatment of C. auris shunt infection with intraventricular caspofungin. Medical Mycology Case Reports. 2018; 22: 35-37.
- Lingas E, Lucio Paredes MM, Jahan M, et al. A case of Candida auris candidemia in an immunocompetent traumatic brain injury patient post ventriculoperitoneal shunt and peripherally inserted central catheter line. Cureus. 2020; 12(6): e8850.

