Rare Association of Wide Cia And Duo Duct in a 30-Year-Old Man: About A Case and Literature Review
Sayarh S, Bouazaze M, Benjelloun H, Fellat N, Fellat R
Department of Medicine, Mohamed V of Rabat, Morocco
Received Date: 09/07/2021; Published Date: 28/07/2021
*Corresponding author: Salma Sayarh, Department of Medicine, Mohamed V of Rabat, Morocco
Introduction
Interatrial Communications (IC) are, afteraortic bicuspidi, the most frequent heart defects: 10% of heart diseases diagnosed at birth, and 30 to 40% of those detected in adults [1]. Embryologically, the inter-atrial septum is formed from 3 structures thati willmerge: the Septum Primum (SeP) which represents the first part of the inter-atrial septum, it descends from the roof of the atrium, the vestibular epine (mesenchyma- teuse origin) which comes from lower and advances forward. At the bottom and in frontare thebuds of the atrioventricular canal, dt of the Septum Secondum (SeS) which also descends from the roof of the atrium. In 4/100,000 newborns, a developmental error results in a defect in the interatrial septum [2]. The CIA is due to the presence of a real defect (lack of constitution) of the inter-ear septa.
The population of adult congenital cardiac patients is growing and it is considered that now more than 90% of children born with a heart defect survive their cardiopathy and reach adolescence and adulthood [3]. These congenital heart diseases of the adult, formerly called "GUCH" (Grown-Up Congenital Heart Disease), and currently referred to as, the term "Adults with Congenital Heart Diseases" is now used to be in line with the international literature, in front of the increase in the age of patients, and they include, not only the malformations treated in childhood that recompense secondarily, but also the asymptomatic cardiopathies at birth becoming symptomatic late, sometimes in adulthood.
There are several anatomical forms: CIA Ostium Secondum (CIAOS): the most common form (75%), twice as common in women as in men [4], CIA ostium primum (CIA OP) (15 %) [5], defect near theatrioventricular valves; integrating as part of a partial atrioventricular canal, CIA sinus venosus (CIA SV) (10%) [5] sits at the upper part of the inter-auricular septum (AIS), at the foot of the superior vena cava (VCS) and is often accompanied by a right upper pulmonary abnormal venous return. [4].
Other types of CIA are less common: "low septal defect" type ACI are low, close to the inferior vena cava (VCI), infrequent, and can be the cause of desaturation in adults, and CORONARY SINUS type AECs, dehiscence’s of the roof of the coronary sinus, are even rarer and difficult to diagnose. They can accommodate a left VCS draining into the coronary sinus. [4]
The diagnosis is based on a bundle of clinical arguments from auscultation and physical examination but also paraclinical and the examination of choice for the diagnosis and quantification of THE CIA is the transthoracic echocardiography which makes it possible to visualize the presence of a right ventricular overload and a tricuspid insufficiency and to measure the pulmonary arterial pressures. Transesophageal echo-cardiography is generally reserved for the evaluation of ACI type "ostium secundum" before percutaneous closure, in order to exclude the presence of abnormalities of pulmonary venous return and abnormalities of the venous sinus. Magnetic resonance can serve as an alternative technique if the information obtained by echocardiography is insufficient, and more particularly for the evaluation of the right ventricular overload and the abnormalities of the pulmonary venous return. Cardiac catheterization is reserved for situations with high pulmonary arterial pressures in order to assess pulmonary vascular resistance.
The management of AIC is based on two components: interventional catheterization or surgery under CEC
The choice depends on the type of CIA, note that the percutaneous technique has replaced surgery for five years for Interatrial Communications (CIA) ostium secundum with favorable anatomy and now considered as the reference treatment it must be systematically discussed for ICD ostium secundum. The lower hospitable morbidity of the percutaneous technique encourages to correct earlier the ICD of young subjects, but also to propose a therapeutic solution not very aggressive to a symptomatic elderly population.
Clinical Case
Patient aged 30 years with 10-bp smoking as cardiovascular risk factors, weaned 03 years ago, with no specific history
Admitted for dyspnea and increased volume of the lower limbs.
In whom the clinical examination finds a systolic breath in the pulmonary focus and a duplication of the
B2 , with signs of right heart failure to type of edema of the lower limbs , turgor of the jugular vein and hepato jugular reflux .
At the ECG is noted the presence of a right axial deviation, HVD and HAD.
thoracic X-ray shows cardiomegaly with dilatation of the right atrium and ventricle, a clear protrusion of the pulmonary arterial arch and an appearance of hypervascularisation of the parenchyma.
Objective trans thoracic echocardiography an old congenital heart disease with pre-tricuspid and post-tricuspid shunt type in severe PAH: very wide CIA of 43 *37 * 40 mm that left shunt right and right left without pulmonary venous return abnormalities associated with a large dutonial canal of 16 mm shunting exclusively right left with a very dysfunctional dilated VD and a spontaneous contrast intra OD, a circumferential pericardial effusion of low abundance.
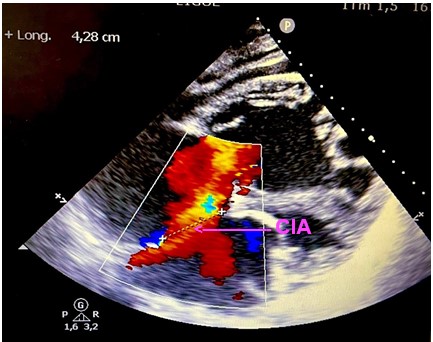
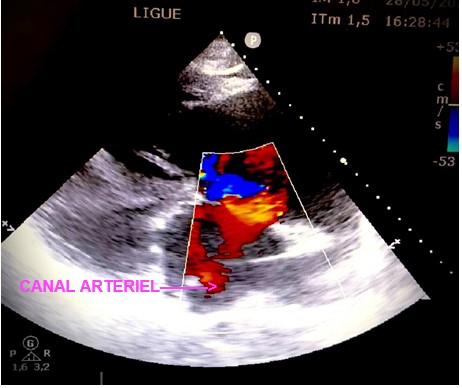
Discussion
Definition
Atrial communication is one of the most common congenital heart defects in adults [6]. There is a clear predominance of the female over the male sex.
Because of its clinical characteristics, the reputed tea behavior in the adult patient has been controversial. Few or no symptoms, good tolerance to physical exertion and long survival were factors that hinder the therapeutic decision [7][8]
Survival to adulthood is the rule, being that life expectancy is not quite normal, and special health care for adults with congenital heart disease is presumed necessary [8]. Pathophysiology
Before birth
Due to the peculiarities of blood circulation in the fetus, there is physiologically a wide communication between the right and left atria: the foramen oval. By this communication, oxygenated blood coming from the placenta through the umbilical vein is directed preferentially to the brain of the fetus via the left atrium, the left ventricle and the ascending aorta. This important supply of blood is also necessary for the development of the structures mentioned above. In his absence, they would only have to carry minimal blood flow from the lungs, which is only 7% of fetal cardiac output.
The presence of communication between the auricles is therefore physiological and necessary before birth.
At birth
Normally this communication closes. The persistence of inter-ear communication is abnormal and results from:
- either the absence of closure of the foramen oval, called permeable foramen oval FOP or PFO - patent foramen oval - in Anglo-Saxon), it is not strictly speaking a "heart defect" but the persistence of a fetal physiological structure;
- or the presence of a real defect (lack of constitution) of the inter-ear septa, called "inter auricular communication. The inter-ear communication communicates two chambers with low resistance, the left and right atria. The left-right shunt in cases with a restrictive septal defect depends on the diameter of the permeable foramen and the pression gradient between the two atria. The difference in the diastolic tally pressures of the two ventricles also participates to a lesser extent. Holes of less than 0.5 cm2 are accompanied by small short circuits while those of 2 cm or more in diameter evolve with a large short circuit.
In wide inter-auricular communications, case of our patient, the left-right shunt depends exclusively on the difference in diastolic tally pressure between the two ventricles, and for this reason the passage of saturated blood from the left atrium to the right atrium occurs during ventricular diastole. This mechanism is clearly observed when we use color Doppler in the diagnosis of inter-ear communication. The fact of communicating circuits with low resistance makes pulmonary arterial hypertension in this pa tantalate. Pulmonary arterial hypertension in patients with hyper pulmonary flow, as occurs in inter-auricular communication, has as its starting point the lesion suffered by the endothelium, which produces a fragmentation of the subendothelial barrier, and the presence of a serous factor which does not normally exist in this surface. This serous factor activates an enzyme which, released from precursor or mature smooth muscle cells, activates growth factors and produces hypertrophy, proliferation and migration of these smooth muscle cells and, later, the proliferation of intima [9]
A shunt is considered significant when the Qp/Qs (ratio between systemic flow and pulmonary flow) is greater than 1.5 or when it causes dilatation of the right cavities.
This mechanism evolves slowly and is mainly due to the court-circuit between the chambers with low resistance, unlike other septal defects, such as ventricular communication. The onset of right ventricular hypertrophy is late and, therefore, the decrease in the left shunt to death in cases of pulmonary arterial hypertension begins when right ventricular hypertrophy is accompanied by an increase in tele diastolic pressure of this chamber. As the tele diastolic pressure increases, the shunt is gradually reduced until it reverses, resulting in the appearance of cyanosis.
This pathophysiological behavior explains why in adult patients we find cases of atrial communication with shunt from left to right, cardiomegaly with pulmonary hyperflux.
In these patients, high pulmonary vascular resistance exists with a still normal right ventricular tele diastolic pressure.
In adulthood, there are comorbidity factors that change the pathophysiology of atrial communication. If congenital heart disease is associated with ischemic or hypertensive heart disease, an increase in the shunt from left to right is observed, produced by the increase in tele diastolic pressure of the left ventricle. When this behavior occurs with pulmonary arterial hypertension, a cardia deficiency that right appears. In the absence of pulmonary arterial hypertension, left ventricular diastolic dysfunction increases pulmonary flow, episodes of airway infection are more frequent, and physical limitation appears.
The application of cyanosis is progressive, accompanied by polycythemia and hypoxemia, the severity of which will depend on the degree of the shunt and the time of evolution. At this stage, patients are very symptomatic, they may develop pulmonarias or cerebral infarctions and platelet dysfunction, among other complications of polycythemia or hypoxemia.
Types the CIA
The majority of atrial communications in adults are localized in the oval fossa; This septal defect is known under the name of ostium secundum. Much less frequently, it is located in the upper part of the atrial septum and, generally, with a partial abnormal connection of the right pulmonary veins, being known as the "venous sinus", or in the lower part of it, called ostium primum. Two-thirds of in-ear communications are ostium secundum and are associated with partial abnormal connection of the pulmonary veins and prolapse of the miracle valve.
- CIA ostium secundum type: 75% of ICDs discovered in adulthood, with locations and sizes and
varied morphologies. Located right in the inter-atrial septum. at the level of the oval pit. Related to the absence of closure of the ostium second (located in the septum primum). It is very well visible in cardiac ultrasound in four cavities, parasternal small axis or sub-costal. The left-right color shunt is better visible in small axis or sub-costal because in 4 cavities, the flow is often perpendicular to the shunt and more difficult to see. It is important to identify the presence of ledges when considering percutaneous closure, especially in the posterior and lower.
- - CIA type ostium primum: 15% they are located outside the oval pit at the edges of the valves
atrioventricular and fit within the framework of a partial or complete atrioventricular canal. The diagnosis is made on the cut 4 cavities where one visualizes an alignment of the mitral and tricuspid valves in the same plane (absence of mitro-tricuspid shift) and an anterior and low inter-auricular communication, glued to the AV valves. It is associated with a cleft of the mitral valve at the level of its anterior leaflet that can be complicated by a mitral insufficiency more or less important.
- CIA sinus venosus 10% of adult ICD. This is a dehiscence of the partition separating the veins
pulmonary of the superior vena cava; the CIA is high located and is often associated with a right upper partial abnormal pulmonary venous return which should always be sought in practice!
- CIA of the coronary sinus: Rare, secondary to a defect of formation of the roof of the coronary sinus with for
consequence a partial or total avouchment of the coronary sinus in the left atrium allowing a left-right shunt through this perforated coronary sinus (unroofed coronary sinus).
- - CIA type low septal defect or low CIA: they concern the lower part of the interatrial septum
close to the inferior vena cava.
Diagnostic
Patients with CIA are often asymptomatic until adulthood and symptoms often include a reduction in functional abilities, dyspnea or palpitations revealing supraventricular tachycardia. Pulmonary arterial pressures increase with age, but the development of severe pulmonary vascular disease is rare. Cyanosis or paradoxical embolism can complicate a CIA shunting D-G.
When the CIA is wide with a very complete right ventricle and/or a poorly compliant left ventricle, the shunt is important and the CIA may be less well tolerated.
Most often, signs of intolerance appear only in adults. Fixed pulmonary arterial hypertension (PAH) may occur between 20 and 30 years of age, and functional intolerance with heart failure and rhythm disturbances (flutter and atrial fibrillation) may occur around 30 to 40 years of age.
More rarely, it is in the face of complications of THE CIA that the diagnosis is made: repeated pulmonary episodes and more exceptionally heart failure in an infant; in this last case the early revelation of heart disease must make look for an associated abnormality in particular anabnormalpulmonary venous reto your [10].
Chest X-ray may show: cardiomegaly by dilatation of the right cavities, with a protrusion of the right lower edge (OD) and the left middle arch (AP), as well as a pulmonary hypervascularization.
The electrocardiographic image shows signs of right ventricular overload with a large R'wave and a deviation of the cardiac axis to the right, a left axis must make suspect a CIA type ostium primum.
However, this is neither specific, nor constant. In children, it can remain quite normal in the presence of a small CIA and usually remains so in the presence of a permeable foramen oval. Usually, there are signs of diastolic overload of the right heart cavities: Hypertrophy of the right atrium (fickle), deviation to the right of the electrical axis of the heart (frequent), appearance of right incomplete block (frequent). When the shunt is actually large may appear more frank signs of hypertrophy ofthe right ventricle. In adults, the ECG may be normal. It can also show hypertrophy of the atrium and right ventricle, lengthening of the PR interval [11]. There is often the presence of atrial rhythm disorders: extra-systo the auricular or atrial fibrillation in advanced forms. It should be noted that in case of BAV 1 associated it is necessary to think about the familial form associated with a mutation of the NKX 2.5 gene.
Trans thoracic echocardiography remains the key examination to make the diagnosis of the type and size of the CIA, the number of defectors, the diameter of the defect and appreciates the impact on the right cavities: dilatation, evaluation of lung pressures, Qp / Qs.
The doppler makes it possible to visualize the shunt and measure the pressure difference between the two atria by measuring the speed of the flow.
It also makes it possible to look for the associated lesions, to evaluate the therapeutic possibilities, in particular percutaneous closure. The study of the inter-auricular septum and pulmonary venous returns is not always easy in trans-thoracic ultrasound, the trans esophageal ultrasound can complete this examination, but it is generally reserved for the evaluation of the ACI type "ostium secundum" before percutaneous closure by specifying the anatomy of the lesions and measuring the banks, it also allows to exclude the presence of abnormalities of the pulmonary venous return and abnormalities of the venous sinus, it then takes all its interest in the septum inter atrial and pulmonary venous returns being better seen in this incidence.
MRI is the baseline examination for measuring the size of the right ventricle and evaluating its function. It is also the examination of choice to rule out an associated abnormality of pulmonary venous returns. For patients contraindicated to MRI, CT scan or cardiac catheterization can bring the missing information to echocardiography. For technical reasons, ETO is not applicable to young children. But MRI seems interesting in this indication, especially in the analysis of pulmonary venous returns [12]
Indications of hemodynamic exploration before the surgical cure of CIA have become rare and concern mainly cases where there is a discrepancy between the clinical picture and the data of the ultrasound examination [13]: the importance of the shunt is not explained by the size of the CIA or it cannot be highlighted.
Right catheterization is no longer used for diagnostic purposes but is indicated in the rare cases where pulmonary pressures could not be determined at the doppler, or when these pulmonary pressures are high, in order to assess pulmonary vascular resistance.
A precise hemodynamic evaluation of the pulmonary resistances and the degree of shunt is necessary in this case before considering a closure of the defect.
Diagnosis is based on one or more of the following abnormalities: Abnormal catheter path, staged oximetry data, indicator dilution curves and angiography:
-The passage of a catheter of the right atrium in the left or eillette can reflect both the presence of an inter-auricular communication with shunt and that of an oval foramen without shunt. In the right atrium, an enrichment of two volumes of O2 per 100 ml of blood compared to athetotic value recalculated from the contents of the VCS and the VCI is necessary to affirm the shunt.
-At the oximetry, the quantification of a shunt requires the calculation of the pulmonary flow (QP), the effective pulmonary flow (Qpe) and the systemic flow (Qs).
The operative pulmonary flow represents the amount of peripheral venous blood subjected to hemostasis each minute. It is calculated using the arterio-venous difference between pulmonary venous blood (CVP) and blood taken from the cavity immediately upstream of the shunt which represents the mixed venous blood (Cv), by the vena cavas for the CIA
Qpe = VO2 / (Cvp-Cv).
-Angiocardiography
Clouding of the heart cavities is one of the safest ways to detect or confirm intra-cardiac shunts. The detection of a G-D shunt requires a selective injection of the contrast medium into the cavity from which the shunt originates
-The left-right shunt can be detected by two types of dilution curve.
- arterial dilution curve: The shunt causes two disturbances that modify the curve:
- an increase in the pulmonary blood volume responsible for a decrease in the initial peak concentration.
- the appearance of a peak of premature recirculation related to the shunt that breaks the descending part of the curve.
-By making staged injections, it is possible to locate the shunt: the first cavity where the dilution curve is normal is located immediately downstream of the pathological cavity. This technique can detect a shunt of more than 20% of the lung flow.
- dilution curve vein uses which allows:
- affirm the shunt by showing an early peak concentration preceding the peak of maximum concentration (ascending portion of the curve),
- to specify the level of the shunt: the early accident is present in the cavities located at the levelor downstream of the shunt, and absent in the cavity located upstream.
- detect a left-right shunt representing more than 5% of lung flow.
The evolution of AIC in childhood is in the vast majority of cases without incident and children can lead a normal life with normal physical activity [14]
Therapeutic Conduct
A spontaneous closure is possible for small ICAs [15]. The management of ICD is based on two components: interventional catheterization or surgery under CEC. The choice depends on the type of CIA: Isolated CIA OS, when anatomy allows, must be closed percutaneously. All other forms of CIA are surgically closed. It should be noted that the CIA entails an over-risk of mortality, whether it has been closed or not, but its closure makes it possible to reduce this risk without making it equal to that of the general population.
-The percutaneous closing of the CIA has evolved over the last 30 years, with the advent of various devices; the Ampliated prosthesis remains the device of choice, approved in 2001 by the "US Food and Drug Administration" for the percutaneous closure of CIA OS [16]. Percutaneous closure was considered technically possible in 80% of patients with CIA OS [17].
Percutaneous closure of CIA ostium secundum showed a clear superiority over surgery
, recently 2 studies have shown that the all-cause mortality rate for percutaneous CIA closure was lower than that of surgical closure (0.09% versus 0.13% and 0.60% versus 0.88%) [18]. and is carried out by femoral venous route, by means of a prosthesis with double disc. Several anatomical conditions of these CIA OS are required, including [maximum CIA diameter + 14 mm] < total length of the AIS (measured in apical 4 cavities), CIA banks > 5mm (optional in retro-aortic) and if possible, a non-floppy septum (indeed, on a very flexible, very aneurysmal rim, the prosthesis may not hold and there is then an embolic risk). The gesture is followed by a platelet anti-aggregation of at least 6 months, the duration necessary for a co-end the location of the prosthesis. Complications are rare, and by argument of frequency: hematoma at the puncture point, embolism or retro-aortic lesion (in the case of very large CIA with prosthetic sizes > 40 mm).
The percutaneous closure is therefore:
- Indium for ICD Ostium Secundum with ledges of at least 5 mm (except towards the aorta), whose stretched diameter does not exceed 38 mm (case of 80% of patients) [19] with left-right shunt, and normal or high pulmonary pressures without Eisenmenger reaction.
- Possible for CIA with right-left shunt in patients with Epstein’s disease. [20]
It should be noted that a CIA OS with an expansion of the tricuspid ring must also be closed percutaneously. It was shown a spontaneous decrease in the diameter of the ring after closure.
And in the particular cases of THE CIA of the elderly subject or "aged CIA", the discovery of a CIA with dilatation of the right cavitates in the elderly subject with dyspnea must lead to the closure of the septal defect because it allows the improvement of the symptomatology and the improvement of the prognosis in the long term. After 40 years, the closure of the CIA does not decrease the risk of supraventricular arrhythmia. Thus, it is important to discuss in the elderly subject the advisability of a gesture of ablation by radiofrequency before the percutaneous closure of the CIA.
-Surgical repair is associated with low mortality (1% chez patients without significant comorbidity) and gives good long-term results (normal life expectancy and low long-term morbidity) especially when it is carried out in the absence of pulmonary arterial hypertension in adolescents and young adults.
In the adult population of advanced age and in the presence of comorbidities, surgical repair seems, on the other hand, to be linked to an increase in mortality [21].
The closure of the CIA prevents the occurrence of heart failure, but if the shunt is closed late (beyond the age of 35), it does not prevent the occurrence of atrial rhythm disorders and strokes. On the other hand, a closed CIA in childhood is generally considered to be hemodynamically cured, but remotely exposes to the risk of rhythm disorders by re-entering around the scar or the patch of farm.
Surgical closure can be done by classic median sternotomy, right summary thoracotomy (preferred in teenagers or adult women) or right post-lateral. It is reserved for ICD: Type ostium Primum, sinus Venosus, coronary sinus and Lowseptal defect when the shunt rate is significant, with a pulmonary flow double the aortic flow (Qp / Qs>2) according to the American recommendations of the AHA / ACC see Qp / Qs>1.5 [22] according to the European recommendations [23].
The indications of closure for CIA, according to the European and American recommendations are respectively summarized in the following two tables:

- In adults with isolated secundum ASD causing impaired functional capacity, right etrizd and/or RV enlargement, end net left-to-right shunt sufficiently large to ceuse physiological sequelae (eg., pulnusnary- systemic blood flow ratio [Qp:Qs] II.5:1) without cyan0sñi at zest or duri exercise, transcatheter or surgical dosure to re&Joe RV volume md improve exercise tolerance is recommended, provided that systolic PA pressure is less than Sgt of systolic systemic pressure end pulmonary vascular resistarxe is less than one third of the systemic vasculw res”tstance (54.1.1-7 1.1-12]. 5. 5. Adults with primum J\SD, sinus venasus defect or coronary sinus defect sequelee (eg., ap:Es *1.5:1] without basis at rest or Amis exercise, shotJld J¥e surgiczzfly' Tepeñed wJess predluded by comorbidrbes, provided that systolic PA gesture is less than 5@/6 of systerrñc gesture end pulmonary vascular resi6tanoe is less the one third of the systemic vascular res”stance (54.11-13, S•'L1.1-14].
- In a6ymptorruztic edults with isolated secundum ASD, r@ht atrial and RV enlargement, arm net left-to-r@ht shunt sufficiently large to cause physiolagical sequelae [e. , Ctp:€ts 1.5:1 or greater], without cyanoais at rest or during exercise, transcatheter or surgical dosure is reasonable to reduce RV volume end/or inn ove functional capacity, provided that systolic PA pressure is less than 50% af systemic pressure and pulmonary vascular resistance is less than one third systemic resistance [S4.1.1-7].
- Surgical dosure of o secundum ASD in adults is reasonable when a concomitant szJrgical procedure is being performed ond there is a net left- to-right shunt sufficiently large to cause physiological sequelae [e. Qp:Qs 15:1 or greater] and right atrial ant RV enlargement without cyenosis at rest or duriug exercise [S4.1.1-15-Sig.11-18]. Percutaneous or surgical dosure may be considered for adults with ASD when net lefi-to-right shunt (Qp:Qs) is 1.5:1 or greater, PA systolic pressure is 50% or more of systemic interim systolic precaure, and/in pulmonary vascular resistance is greater than one third of the systemic resistance(54.1.1-19, 11.1-20].
- ASD dosure should not be performed in adults with PA systolic pressure greater then two thirds systemic, pulmonary vascular resistance greater than two thirds systemic, and/or a net right-to-left shunt (54.1.1-21, 54.1.1-22).
According to ESC Recommendations 2020 in the management of congenital heart disease in adulthood, it is noted that in patients with shunt and non-invasive signs of PAP elevation, it is recommended an invasive measurement of PVA (Fick's method)
- Pre- and post-tricuspid shunts are individualized
- Regarding the closure of shunts (when Qp/Qs > 1.5) according to the calculation of THE RVP
- <3UW: closure for CIA (classI)
- 3-5UW: closure for CIA (classIIa)
- ≥ to 5UW but <5UW after treatment of HTP: fenestrated closure for ICD (classIIb)
- ≥ to 5UW: it is not recommended to close the CIA (classIII)
- The percutaneousclosure of the CIA OS is the method of choice if technically accessible (class I)
- In patients with CIA and VG disease, it is recommended to do aballoon occlusion test and evaluate the benefit of shunt closure versus the risk of its closure with
increase in filling pressures (closure, fenestrated closure or no closure) (Class I) aftercare.
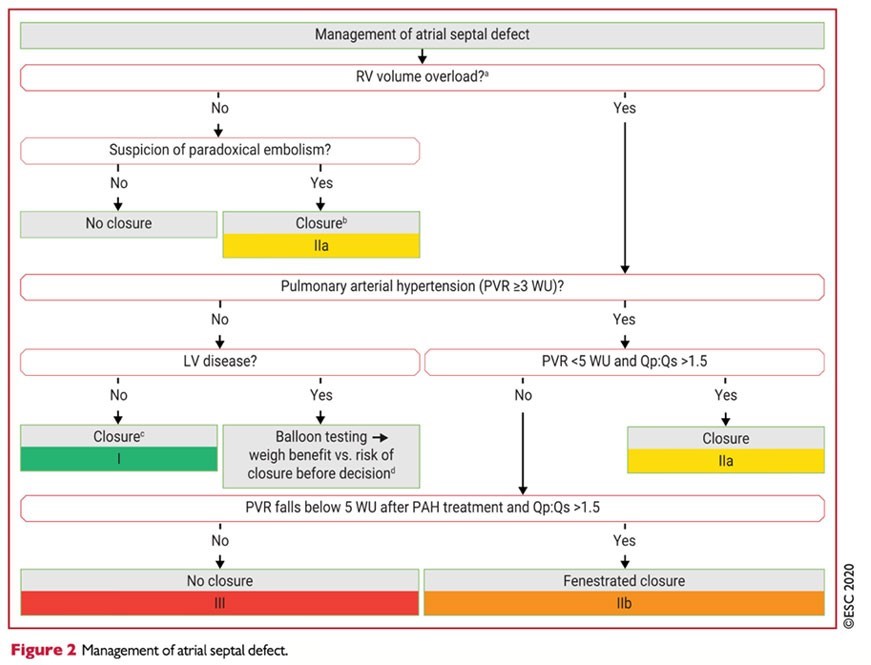
The surveillance of a CIA operated in childhood or before the age of 25, justifies, in the absence of functional symptomatology or PAH, only a control every 4 or 5 years. [24] All other patients must be regularly monitored, including an assessment in a specialized center.
«GUCH».
After closure, the follow-up by echocardiography makes it possible to check the tightness of the patch, or persistence of a residual shunt, the adequate positioning of the Amplatzer prosthesis, to evaluate the function and dimensions of the right and left ventricles as well as the pulmonary arterial pressures, and to identify the presence of tricuspid insufficiency.
The anamnesis, the electrocardiogram and, in specific cases, a Holter-ECG, will be useful for the detection of arrhythmias, knowing that:
*After surgical closure before the age of 40, it is mainly tachycardias [25]
by intra-atrial re-entry or atrial flutter. They can be treated by radiofrequency ablation.
*An UN repaired or repaired CIA after the age of 40 will be frequently complicated by atrial fibrillation and will be treated with antiarrhythmics and anticoagulants.
The American recommendations of AHA / ACC, propose a rhythm of follow-up and realization of examination (ECG, ETT ...) according to the physiological stage of the patient [22]
Conclusion
More and more children with congenital heart disease are reaching adulthood. The diagnostic approach, therapeutic management and follow-up of congenital pathologies most frequently encountered in adulthood have been the subject of recommendations by the European Society of Cardiology. Given its great specificity and the diversity of anatomy-clinical situations, this population most often requires care in specialized units.
References
- Ward C Secundum atrial septal defect: routine surgical treatment is not of proven benefit. Br Heart J71, 1994; 219223.
- Lindsey JB, Hillis LD. Clinical update : a trial septal defect in adults. Lancet, 2007 ; 369: 1244-1246.
- Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival into adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010; 22: 2264-2272.
- Vaksmann G, Richard A. Management of aortic coarctation in adults Private Hospital La Louvière, Vendôme-Cardio, LILLE. Cardiological Realities # 297_ November /December 2013_Cahier General ReviewsVascular
- Cohen S, Iserin L. Congenital heart disease in adulthood. Blood Thrombosis Vessels (2014).
- Dickinson DF, Arnold R, Wilkinson JL. Congenital heart disease among 160 480 liveborn children in Liverpool 1960 to 1969. Implications for surgical treatment. Br Heart J. juill 1981; 46(1): 55-62.
- Campbell M. Natural history of atrial septal defect. Br Heart J 1970; 32:820-825.
- Brickner ME, Hillis D, Lange RA. Congenital heart disease in adults. N Engl J Med 2000; 242: 256-263.
- Rabinovith M. New concepts in pulmonary vascular disease. En: Freedom RM, editor. Congenital heart disease. Philadelphia: Cu- rrent Medicine, 1997; p. 1-10.
- Heitz F. Congenital heart disease. EMC AkOS Practical Encyclopedia of Medicine 1998; 8:1-14. Auscultation is typical showing a fixed duplication of B2 and a systolic breath at the pulmonary focus, the search at clinical examination for signs of iCDTe and / or PAH, arrhythmia is systematic.
- Bofferding L, Hascoet J. M.Early management of a newborn with or suspected congenital heart disease. Archives de Pédiatrie 2001; 8:1116–20.
- Kastler B, Livolski A, Germain P, et al. MRI of congenital heart disease. EMC Cardiology Angeliology 2005; 2: 27-72.
- Rey C.Treatment of congenital heart disease by interventional catheterization. Pediatric Archives 2004; 11: 639–641.
- Petit J, Losay J, Bouchard F, Issad M, Maribas P, Lucet P. [Hemodynamic development of the auricular communication of the ostium secundum and sinus venosus type. Study of 1189 patients]. Arch Mal Coeur Vaiss. juill 1986;79(8):1162-1167.
- Hanslik A, Pospisil U, Salzer-Muhar U, Greber-Platzer S, Male C. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. Pediatrics. oct 2006; 118(4): 1560-1565.
- Turner DR, Owada CY, Sang CJ, Khan M, Lim DS. Closure of Secundum Atrial Septal Defects With the AMPLATZER Septal Occluder. Circulation: Cardiovascular Interventions, 2017 ; 10(8): e004212.
- Godart F, Houeijeh A. Interventional catheterization in congenital heart disease. La Presse Médicale, 2017; 46(5): 497-508.
- Turner DR, Owada CY, Sang CJ, Khan M, Lim DS. Closure of Secundum Atrial Septal Defects With the AMPLATZER Septal Occluder. Circulation: Cardiovascular Interventions, 2017; 10(8): e004212.
- ESC Guidelines 2010 – guidelines for the management of grown-up congenital heart disease (GUCH). Eur Heart J 2010.
- Vaksmann G, Richard A. Management of aortic coarctation in adults Private Hospital La Louvière, Vendôme-Cardio, LILLE. Cardiological Realities # 297_ November /December 2013_Cahier General ReviewsVascular
- Miltner Seghaye BP. Lancellotti European recommendations for the management of congenital heart disease in adults; (1), M-C. Rev Med Liège (2013).
- Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018.
- ESC Guidelines. Guidelines for the management of grown-up congenital heart disease (GUCH). Eur Heart J 2010.
- Miltner Seghaye BP. Lancellotti European recommendations for the management of congenital heart disease in adults; (1), M-C. Rev Med Liège, 2013.
- Bertrand Dugardin. Access to loan insurance for adult patients withcongenital cardiopatitis. Human medicine and pathology. 2010.

