Left Atrial Myxoma, Mitral Regurgitation and Coronary Heart Disease: A Case Report of an Unusual Association
Zakariae Laraichi*, Larsen Clarck Moumpala Zingoula, Nawal Doghmi, Mohamed Cherti
Cardiology B Department, Ibn Sina University Hospital Center, Mohammed V University, Morocco
Received Date: 02/07/2021; Published Date: 22/07/2021
*Corresponding author: Zakariae Laraichi, Cardiology B Department, Ibn Sina University Hospital Center, Mohammed V University, Boulevard Ibn Rochd, quartier Souissi, Rabat 10100, Morocco. Email: zakariae.laraichi@usmba.ac.ma
ORCID:
Zakaria LARAICHI - https://orcid.org/0000-0002-5782-062X
Larsen Clarck MOUMPALA ZINGOULA - https://orcid.org/0000-0001-6582-7218
Abstract
Background: Cardiac myxoma is the most common type of primary cardiac neoplasm, usually found in the left atrium. It poses several problems including that of differential diagnosis with a thrombus. Often accidentally diagnosed, or during an etiological assessment, it is objectified on echocardiography and only the pathology confirms the diagnosis. Our clinical case reports an unusual association that prompts any clinician to take a comprehensive approach with cardiac myxoma.
Case Summary: A 64‑year‑old male patient was admitted to our department for dyspnea associated with palpitations. Clinical assessment showed a large left atrial myxoma, severe mitral regurgitation on prolapses of the mitral valve posterior leaflet due to ruptured chordae tendinae as well as coronary involvement with proximal stenosis of the right coronary artery. The patient underwent surgical resection of the myxoma, mitral valve replacement by mechanical valve as well as right coronary bypass surgery, with good postoperative evolution.
Conclusion: Left atrial myxoma associated with mitral regurgitation is rare. It would only due to the myxoma either by damage to the mitral valve by preventing the coaptation of the leaflets, or by "Wrecking ball" effect explaining the rupture of the chordae tendinae. The presence of coronary artery lesions on angiography in these patients can be due to associated coronary artery disease or due to embolization of tumor fragments into the coronary artery.
Keywords: Left atrial myxoma; Mitral regurgitation; Coronary artery disease; Case report
Abbreviations: LA: Left atrium; TTE: Trans-thoracic echocardiography; TEE: Trans-esophageal echocardiography
Background
Myxoma is the most common benign tumor of the heart, accounting for nearly 50% of primary heart tumors [1,2]. Thanks to the development of cardiac imaging, more and more cases of atrial myxomasare now diagnosed, either incidentally or during a cardiac etiological assessment. The management is surgical by excision of the myxoma from its insertion level [3,4]. We report the clinical case of a patient associating a large myxoma of the Left Atrium (LA), significant stenosis of the right coronary artery, as well as severe mitral regurgitation caused by a prolapse of the posterior mitral leaflet due to a ruptured tendon cord. The final diagnosis was made by histology. This unusual association must be known and researched in clinical practice.
Case Presentation
The patient is a 64-year-old patient, active smoker, admitted for paroxysmal nocturnal dyspnea rapidly progressing accompanied by cough and orthopnea for the past 3 weeks.
On clinical examination, the patient was polypneic, orthopneic, with an irregular pulse, and the presence of a holosystolic murmur at the mitral focus, as well as crackling rales at the pulmonary bases. The ECG showed atrial fibrillation with a heart rate of 150 bpm.
Trans-Thoracic Echocardiography (TTE) showed a rounded mass, with regular contours, of heterogeneous echo structure, measuring 42 x 53 mm, attached by a pedicle to the roof of the left atrium, resembling a myxoma (Figure 1). We found a severe mitral leak caused by a prolapse of the posterior mitral leaflet with a flail appearance suggesting a ruptured tendon cord (Figure 2). Left ventricular systolic function was preserved.
After initial stabilization of the patient by diuretic treatment, a Transesophageal Echocardiography (TEE) was considered for a better morphological approach. The patient presented acute heart failure during the introduction of the probe; we decided to perform it intraoperatively (Figure 3,4). Preoperative coronary angiography showed an intermediate lesion of the proximal left anterior descending coronary artery and a significant stenosis of the proximal right coronary artery (Figure 5).
The therapeutic strategy consisted of a successful surgical excision of the mass (Figure 6); a replacement of the mitral valve by a mechanical prosthesis as well as a bypass graft to the right coronary artery. Histopathological examination confirmed the diagnosis as a benign cardiac myxoma (Figure 7,8). To date, the outcome was favorable without symptoms of dyspnea, and without recurrence.
Timeline
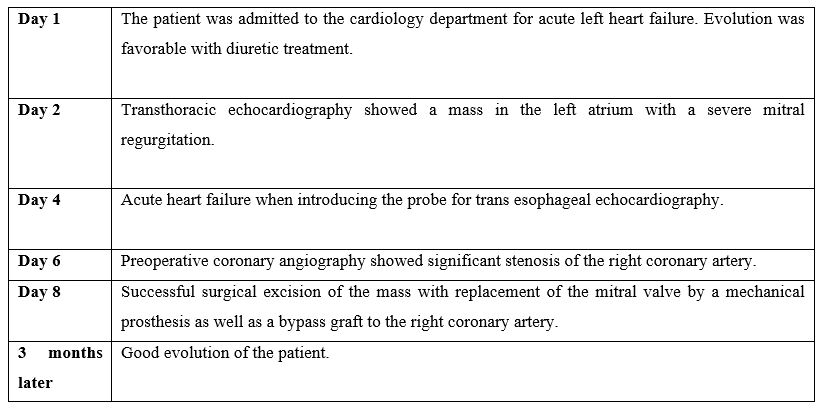
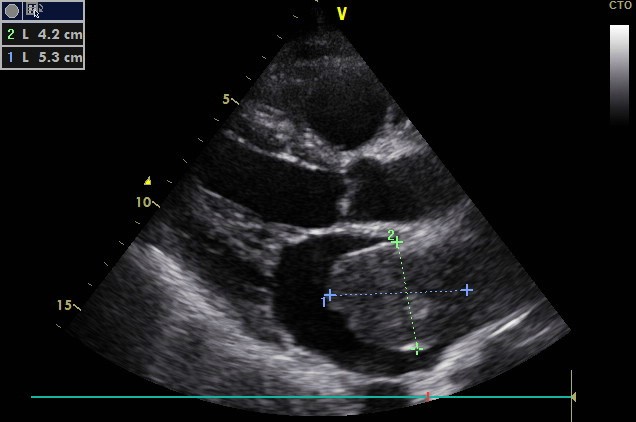
Figure 1: Image of long axis parasternal view in TTE showing a mass in the left atrium measuring 42 x 53 mm.
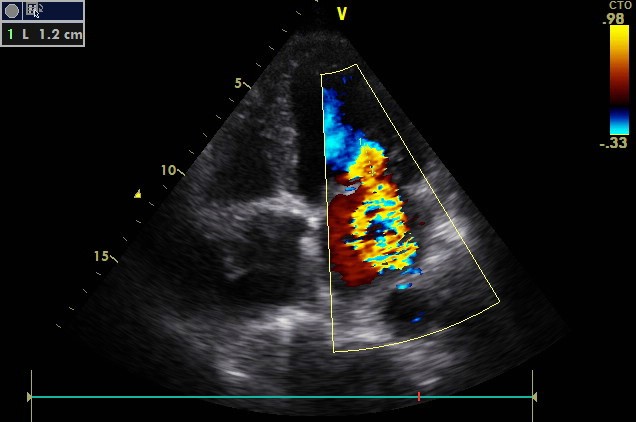
Figure 2: Apical four chamber view in TTE showing severe mitral regurgitation (PISA radius = 12 mm).
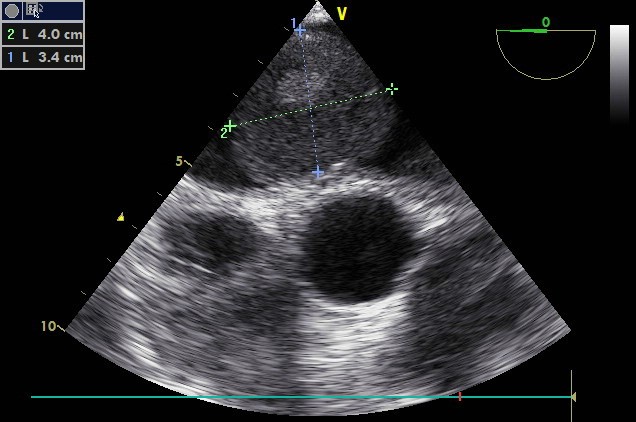
Figure 3: 0° Transesophageal Echocardiography (TEE) image revealing a rounded mass with heterogeneous echo structure.
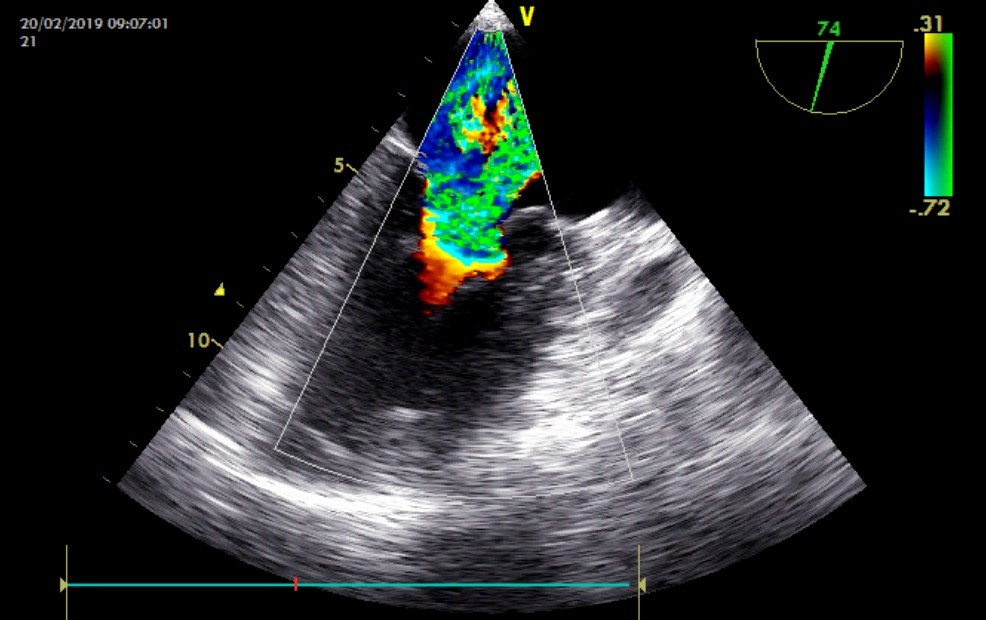
Figure 4: 74 ° Transesophageal Echocardiography (TEE) image showing severe mitral regurgitation.
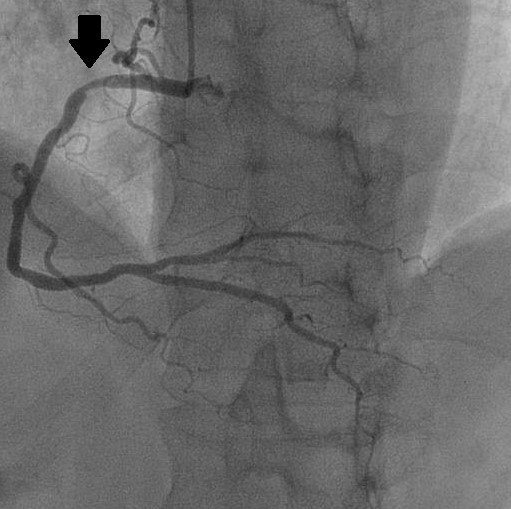
Figure 5: Coronary angiography in Left Anterior Oblique (LAO) projection objectifying a tight stenosis of the right coronary artery at the level of his upper knee (black arrow).
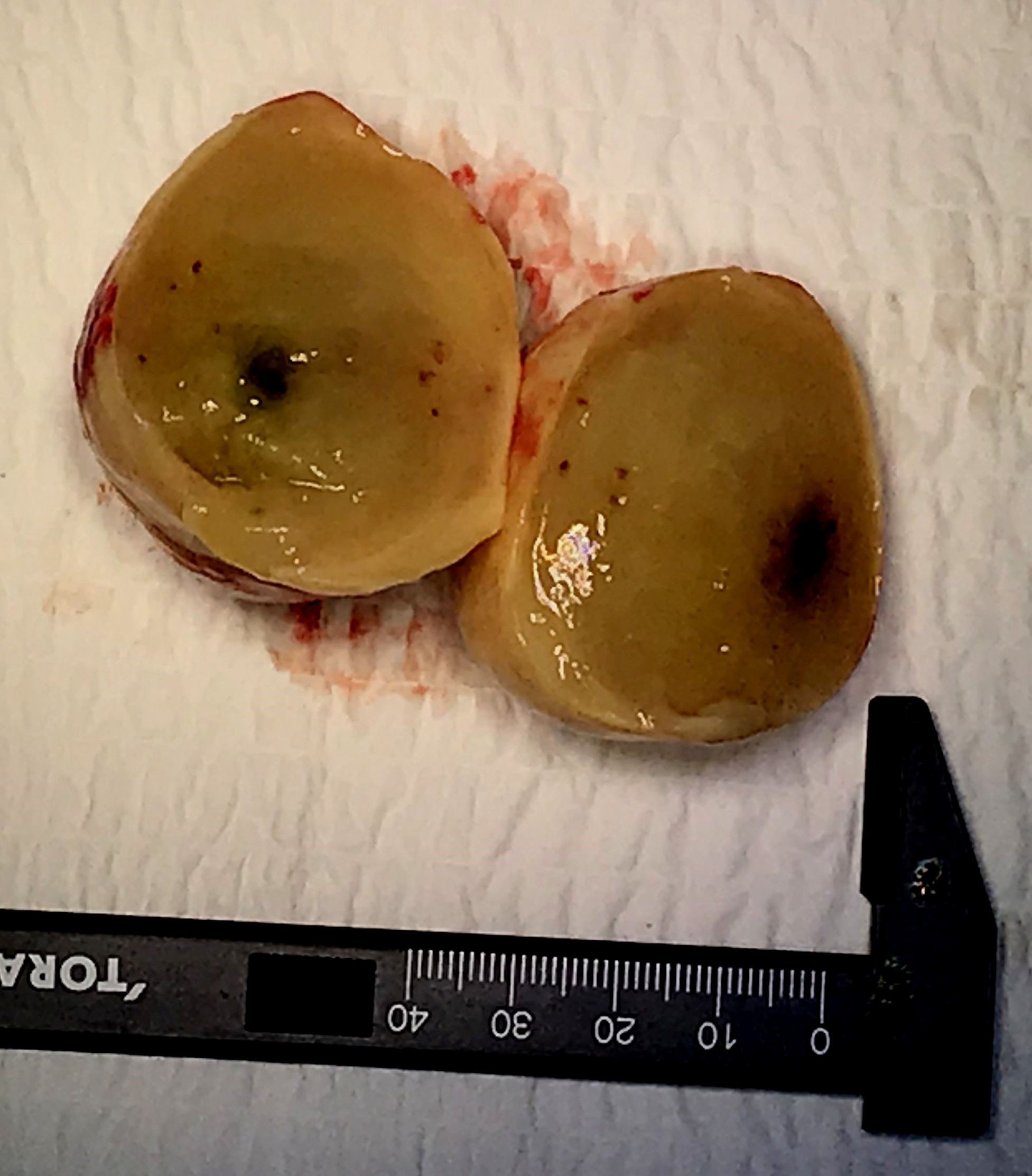
Figure 6: Image of myxoma after resection with the cut section (presence of central necrosis).
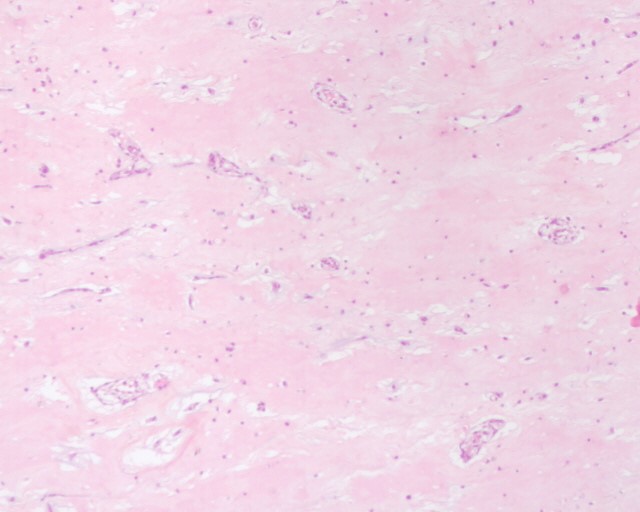
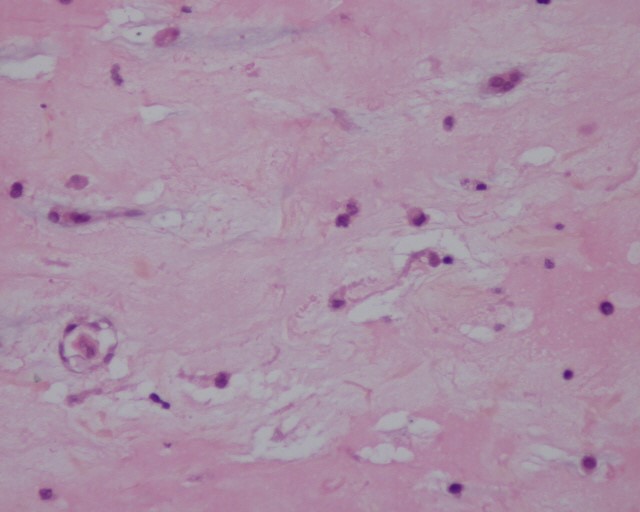
Figure 7, 8: Microscopic appearance of myxoma after Hematoxylin and Eosin (HE) staining × 10 and × 25 respectively: Presence of cells with an ovoid nucleus and abundant eosinophilic cytoplasm and imprecise limits, mixed with mononuclear inflammatory cells organizing around the vessels on a myxoid background.
Discussion
Myxoma is the most common benign heart tumor. Predominant in the left atrium, it is generally a papillary mass with an irregular friable surface, less often ovoid and smooth. They can also be located, in descending order of frequency, in the posterior atrial wall, the anterior atrial wall and the atrial appendage 5. This poses differential diagnostic problems with intracardiac thrombus following atrial fibrillation or myocardial infarction. LA thrombus is usually attached to the LA appendage or, the posterior LA wall by a broad base, usually immobile and is not associated with systemic constitutional symptoms [5]. In our patient, the myxoma was round with regular contours and an implantation base at the roof of the left atrium, which was unusual.
From a hemodynamic point of view, its volume and its mobility during the phases of the cardiac cycle can cause pulmonary congestion: either by obstruction of the admission chamber during diastole [6], or by limiting the emptying or filling of the LA [5,8]. This explains the acute heart failure that our patient presented when doing TEE.
LA myxoma associated with mitral regurgitation remains rare. It is responsible either by preventing the coaptation of the mitral leaflets, or by a "Wrecking ball" effect explaining the rupture of chordae tendineae [5,7,9]. In our patient, we suspected a cord rupture, but without real proof on imaging. The surgeon was able to confirm the rupture of one of the cords of the posterior mitral leaflet intraoperatively. The mechanism therefore remains mysterious: a rupture by acute mitral regurgitation was ruled out in the absence of coronary embolization, and chest pain; and the myxoma did not prolapse through the mitral orifice, thus causing damage to the cords.
In addition, the associated lesion of the right coronary artery in our patient is caused by an atheromatous disease given the nature of the lesion, the cardiovascular risk factors, the absence of an acute presentation in favor of myxoma embolization and finally the type of the mass. Gerasimos Gavrielatos [10] defines two types of myxomas: « a round type typified by a solid and round shape with a nonmobile surface, and a polypoid type characterized by an asymmetrical and soft shape with a mobile surface» [11]. The latter type is a potential source of systemic embolism occurring in a third of myxomas [5,7]. Kumar et al [5] found that the prevalence of coronary artery disease with myxoma ranges from zero to as high as 20.3-36.6%, depending on age and associated risk factors for atherosclerosis. The incidence of coronary embolization of myxomas was 0.06%. According to Sugimot et al [12], embolization seems to depend on the angle between the aortic blood flow and the coronary artery responsible for the infarction.
The management of our patient was a success. He was in total agreement with the management procedure. Open-heart surgery led to an excellent result. Histological study of the operative specimen confirmed the diagnosis and described cells with an ovoid nucleus and abundant eosinophilic cytoplasm with imprecise boundaries, mixed with mononuclear inflammatory cells organized around the vessels. To date, the outcome was favorable without symptoms of dyspnea, and without recurrence.
Conclusion
Developments in cardiac imaging led to increasing number ofdiscovered cardiac masses, frequently myxomas of the left atrium. These benign tumors represent a challenge with both diagnosis and complications. The association of myxoma and coronary lesions is rarely reported, hence the interest in this topic. Any clinician should think about this combination and perform a careful assessment of the mitral valve and subvalvular system.
References
- Reynen K. Cardiac myxomas. N Engl J Med1995; 333: 1610-1617.
- Paulsen W, Nolewajka JA, Boughner RD, Kostuk WJ, Heimbecker RO, Mckenzie FN. Left atrial myxoma: report of six cases and review of the literature. CMA Journal 1980; 123: 519-522.
- Markel ML, Waller BF, Armstrong WF. Cardiac myxoma: A review.Medicine (Baltimore) 1987; 66: 114-125.
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333: 1610–1617.
- Kumar B, Raj R, Jayant A, Kuthe S. Left atrial myxoma, ruptured chordae tendinae causing mitral regurgitation and coronary artery disease. Ann Card Anaesth 2014; 17: 133-136.
- Gallo M, Trivedi JR, Protos AN, Slaughter MS. Massive left atrial myxoma induced congestive heart failure. J Card Surg. 2017; 1-2.
- Laurent Pinede, Pierre Duhaut, et Robert Loire. Clinical Presentation of Left Atrial Cardiac Myxoma: A Series Of 112 Consecutive Cases. 2001; 80: 159-172.
- Vinod Namana, RajeswerSarasam and al. Left atrial myxoma. QJM Advance Access published July 10, 2016.
- JOE R. Wise. Mitral Regurgitation due to Rupture of Chordae Tendineae by Calcified Atrial Myxoma. British Medical Journal, 1974; 2: 95-96.
- Gerasimos Gavrielatos et al. Large left atrial myxoma presented as fever of unknown origin:a challenging diagnosis and a review of the literature. Cardiovascular Pathology 2007; 16: 365-367.
- Demir M, Akpinar O, Acarturk E. Atrial myxoma: an unusual cause ofmyocardial infarction. Tex Heart Inst J 2005; 32: 445-447.
- Sugimoto T, et al. The Problems of Surgical Treatment for Cardiac Myxoma and Associated Lesions. Jpn J Surg, 1994; 24: 673-680.

