Urolithiasis in a 13-Month-Old: A New Case of Adenine Phosphoribosyl transferase Deficiency
Marie Adrianne Pimentel, Anita Lala, Stephen Du Toit, Richard MacKay, Udaya Samarakkody
Department of Paediatric Surgery, Waikato Hospital, New Zealand
Department of Paediatrics, Tauranga Hospital, New Zealand
Chemical Laboratory, Hamilton, New Zealand
Clinical Biochemistry Unit, Canterbury Health Laboratories, New Zealand
Department of Surgical Science, Pain Clinic, Uppsala University, New Zealand
Received Date: 20/04/2021; Published Date: 04/05/2021
*Corresponding author: SMarie Adrianne Pimentel, Department of Paediatric Surgery, Waikato Hospital, Hamilton 3240, New Zealand. Email: dr.adrianne.pimentel@gmail.com
Abstract
We present the first paediatric case of Adenine Phosphoribosyl transferase (APRT) Deficiency in Australia and New Zealand. This is a rare autosomal recessive disorder of purine metabolism, resulting in 2,8-dihydroxyadenine crystalluria associated with recurrent urolithiasis and significant risk of renal impairment, often requiring a combined surgical and medical approach. A 13-month-old girl presented with sepsis secondary to obstructive urolithiasis and was subsequently diagnosed with APRT deficiency during her first hospital admission. She required both endoscopic and open surgical intervention for stone removal. She was also commenced on maintenance allopurinol, dietary restrictions and a surveillance programme for stone recurrence. We compare her case to existing case studies and examine more recent research looking at alternative medications to allopurinol.
Conclusions: The early manifestations of APRT deficiency in this case provide valuable insight into aspects of early diagnosis and management.
Keywords: Adenine Phosphoribosyl Transferase Deficiency; APRT deficiency; 2,8-DHA; Nephrolithiasis; Urolithiasis
List of Abbreviations
APRT - Adenine Phosphoribosyl transferase; 2,8-DHA - 2,8-Dihydroxyadenine; PUJ - Pelviureteric Junction; IR - Infrared; UTI - Urinary Tract Infection; FTIR - Fourier Transform Infrared Spectroscopy; DMSA - Dimercaptosuccinic Acid; ESWL - Extracorporeal Shockwave Lithotripsy; eGFR - Estimated Glomerular Filtration Rate; bpm - Beats Per Minute; BSA - Body Surface Area
Introduction
We present the first paedatric case of Adenine Phosphoribosyl Transferase (APRT) Deficiency in New Zealand and Australia.
APRT deficiency is a rare autosomal recessive disorder of purine metabolism leading to disordered adenine breakdown. When APRT is deficient, xanthine dehydrogenase converts adenine to 8-hydroxyadenine, then subsequently to 2,8 dihydroxyadenine (2,8-DHA). 2,8-DHA is highly insoluble in urine resulting in crystalluria and urolithiasis. In children, the presentation of urolithiasis includes pain, haematuria, and Urinary Tract Infection (UTI). As 2,8-DHA stones are radiolucent, initial diagnosis of urolithiasis is via ultrasonography [1]. The confirmation of diagnosis is based on stone analysis, 2,8-DHA crystalluria on 24-hour urinalysis and/or null APRT enzyme activity [2,3]. Gene testing for the APRT gene on chromosome 16q24 can be performed [4]. Due to the risk of recurrent stones and renal failure, a combined medical and surgical approach is often required.
Case Report
Miss A presented at 13 months of age with obstructive urosepsis secondary to a 10 mm diameter calculus at the left Pelviureteric Junction (PUJ). She had three days of fever and vomiting, continuing despite 48 hours of oral co-trimoxazole commenced in the community for presumed UTI.
Miss A’s medical and surgical history, growth and developmental milestones were normal. Immunisations were up to date. She is a Caucasian New Zealander born to non-consanguineous parents with no family history of nephrolithiasis.
Initial examination findings of note included temperature of 38.6○C, mild tachycardia (130 bpm) and tachypnoea (Respiratory rate 36 breaths/min). Laboratory tests confirmed raised inflammatory markers and normal renal function. The first urine sample cultured Citrobacter freundii as the predominant organism in a mixed growth.
A urinary tract ultrasound performed on day two showed a dilated left upper pole calyx with echogenic debris, reduced perfusion of the left kidney and associated pyelonephritis. Repeat ultrasound performed three days later for persistent fevers confirmed persistent left calyceal dilatation worst at the upper pole, echogenic debris and a 10-millimetre calculus at the left PUJ [Figures 1a-c]. Right urinary tract was unremarkable.
After transfer to a tertiary surgical centre, she underwent ultrasound guided insertion of left nephrostomy tube. The turbid urine from nephrostomy grew citrobacter freundii, sensitive to both gentamicin and co-trimoxazole.
A nephrostogram performed five days later showed multiple left intra-renal calculi and obstructing ureteric calculi, so she proceeded to open left pyelolithotomy. Intra-operative findings confirmed inflamed left renal parenchyma with multiple fibrinous adhesions to peri-nephric tissue, multiple small calculi measuring up to five millimetres with debris in all poles, intra-parenchymal calcification and a dilated proximal left ureter. Nephroscopy was used to facilitate stone clearance. Stones removed were sent for analysis [Figure 2]. Clearance of all intrarenal stones was confirmed on nephroscopy and intra-operative ultrasound. A left nephrostomy tube, Double J ureteric stent and an indwelling urethral catheter were placed. Follow up ultrasound performed five days later showed a new non-obstructing 2.6 mm intrarenal stone in the right midpole, while two small stones remained in the left upper pole calyx with mild residual dilatation.
Anitibiotic therapy continued throughout, and oral potassium citrate was started while metabolic investigations were completed. Staged removals of the urethral catheter, nephrostomy tube and ureteric stent were all successful during the 10 weeks following surgery.
As IR spectroscopy was unavailable in the local laboratory, stone composition was initially reported as urate (LTA Kidney Stone Analysis Kit, Milano, Italy). This kit uses a wet chemical analysis technique: a technique known to have an inability to differentiate between urate and 2,8-dihydroxyadenine [1,2]. Metabolic analysis of 24-hour urine collection confirmed raised urinary adenine 0.074 mmol/L (normal range: 0.000-0.001), and urinary 2,8-dihydroxyadenine of 0.254 mmol/L (normal range: 0.000-0.001). The original urinary tract stones were sent to a laboratory with Fourier Transform Infrared Spectroscopy (FTIR) and these confirmed stones were 60% 2,8-DHA, 20% uric acid and 20% unknown. All other serum and urinary biochemical investigations were unremarkable.
Definitive treatment with allopurinol, low protein diet and increased oral fluid intake was started and potassium citrate discontinued. Expert advice was obtained from the Great Ormond Street Children's Hospital (United Kingdom), Mayo clinic (United States of America) and National University Hospital of Iceland.
Miss A is now 3 years old and has been registered with The Rare Kidney Stone Consortium Patient Registry [3]. She continues on lifelong allopurinol and completed a one-year course of prophylactic co-trimoxazole. Long-term dietary plans include purine restriction and targeted oral fluid intake (1.5 L/m2 BSA). Her surveillance investigations for nephrolithiasis include six-monthly urinary tract ultrasound with paired urine (2, 8- DHA/Creatinine ratio, microscopy and albuminuria) and blood tests (renal function). Post-operative DMSA renogram was normal. She has not required any further surgery for urolithiasis, but has passed several calculi spontaneously. Her most recent surveillance ultrasound in June 2019 has shown a stable 6 mm non-obstructing left lower pole renal stone, with adequate interval growth of both kidneys.
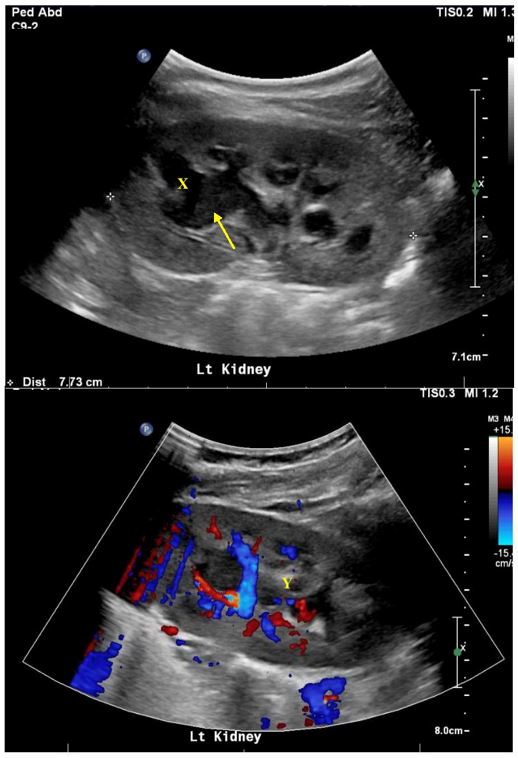
Figure 1: Initial ultrasound confirming left kidney upper pole calyx dilatation (X) with debris (arrow) and reduced perfusion (Y) in keeping with pyelonephritis.
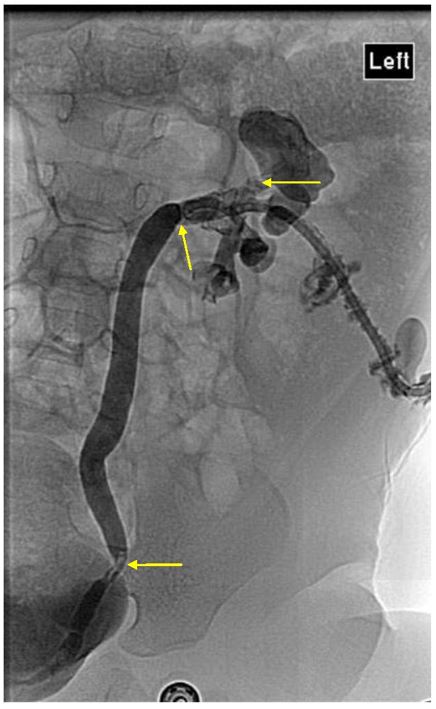
Figure 2: Nephrostogram confirming multiple urinary tract stones, mainly in the left upper pole calyx, mid and distal ureter, with associated urinary tract obstruction.

Figure 3: Intra-operative appearance of intra-renal stone on nephroscopy
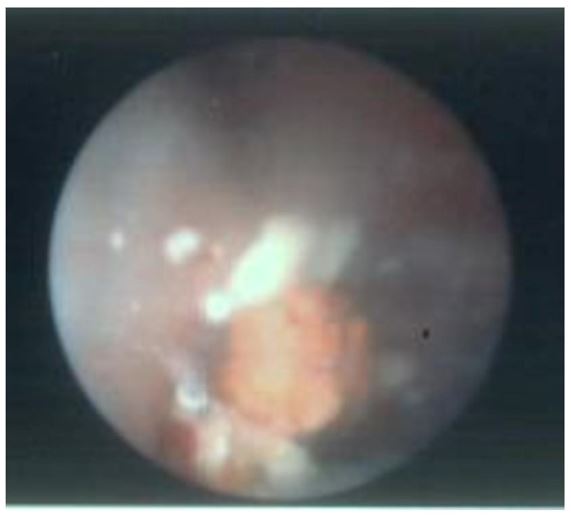
Figure 4: Gross appearance of urinary tract stones
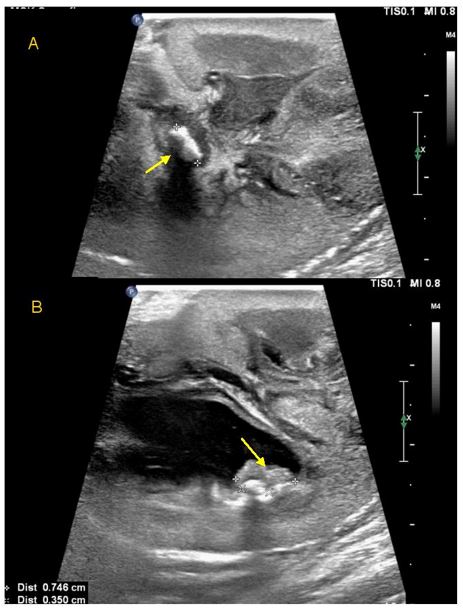
Figure 5: Intraoperative ultrasound images during pyelolithotomy
Table 1 : Initial 24 hour urinalysis results

Discussion
The estimated prevalence of APRT deficiency in Caucasians ranges from 1 in 50,000 to 1 in 100,000, based on current heterozygote rates of 0.4-1.2%. Ceballos-Picot et al argue that due to the much lower number of cases reported compared to the estimated prevalence, APRT deficiency may be under-diagnosed [2]. To date the largest paediatric case series includes 21 patients over a 32 years recruitment period [1]. Diagnosis of APRT deficiency was based on at least one of: 2,8-DHA crystalluria, stone analysis or null APRT activity in blood erythrocytes [1,7-11]. Fortunately, Miss A had a relatively prompt diagnosis in contrast to their study showing 20% of children having a delayed diagnosis, and a median delay of one year. A combined medical and surgical approach is often required, as with Miss A, with a 47% surgical intervention rate reported in one case series [1].
The first case report of APRT deficiency in the English language was published in 1979 by Barratt et al, regarding a 1-year-old girl with a delayed diagnosis of urolithiasis at 21 months after initially being managed for recurrent UTI [7]. Similar to our case, the stone was initially mistaken for a uric acid stone and it was only on further testing that a 2,8-DHA stone was confirmed [7]. This publication reveals a useful description of two important differences between 2,8-DHA and uric acid stones. Firstly, they can be differentiated based on the gross appearance: 2,8-DHA stones are friable, reddish-brown then grey-blue when crushed; while uric acid stones are hard and yellow [7,8,12]. Secondly, the solubility of 2,8-DHA stones does not change with pH changes, so unlike uric acid stones, urine alkalinisation therapy is ineffective. The misdiagnosis of 2,8-DHA stones as uric acid stones, or more accurately, the inability for older stone composition techniques to differentiate between the two, is well-known with some centres referring to external laboratories for analysis [2,10,12,13]. Therefore, the recommendation is that stone composition is analysed using stereomicroscope/IR spectroscopy, rather than biochemical assays [2,12,13]. As this is unavailable in some laboratories, the onus is on clinicians and pathologists to have a high level of suspicion in considering APRT deficiency in any child who presents with "uric acid" stones or any radiolucent stone. However, of note is 2,8-DHA stones can occasionally be radio-opaque if they contain calcium salts [2].
Miss A's treatment with allopurinol and a low purine diet was based on expert opinion and current literature [2,13]. Allopurinol is the mainstay of treatment for APRT deficiency, as the inhibition of xanthine dehydrogenase reduces the formation of 2,8-DHA and hence reduces the chances of stone formation(3). One case series confirmed that stone recurrence is associated with patients who have more frequently positive surveillance crystalluria1. Recently, there has been interest in alternative selective non-purine xanthine oxidoreductase inhibitors. In 2018, Edvardsson et al published an open-label, crossover, non-randomised clinical trial comparing febuxostat to allopurinol in an adult population for APRT deficiency [3]. This study showed that febuxostat (80 mg daily) is tolerated well with no adverse effects and it may be more effective than allopurinol (400 mg daily) in reducing urinary 2,8-DHA, though the difference was not statistically significant and sample size was small (n=8).
Similarly, allopurinol dosing has also been a point of discussion. In the largest paediatric case series to date, patients were treated with allopurinol at a median dose of 9 mg/kg/day, within the range recommended for Miss A [1,2]. A lower paediatric dose of allopurinol was successfully trialled in a Japanese case report published in 2017 [9]. A 1 mg/kg/day dose (rather than the usual 5-10 mg/kg/day) was used in a 30-month-old girl with APRT deficiency, and urinary 2,8-DHA crystals remained undetectable [9]. A lower effective maintenance dose for allopurinol may be an area of research worth pursuing, especially for patients diagnosed in early childhood such as Miss A who is likely to require life-long allopurinol.
Miss A has been registered with The Rare Kidney Stone Consortium Patient Registry [14]. In such rare disorders, such registries are integral for a greater understanding of the natural history of this disease, its relation to other metabolic disorders associated with urolithiasis and managing the risks of long-term renal complications [14,15].
Conclusion
APRT deficiency is a rare autosomal recessive disorder with significant implications for long term renal function and recurrent urolithiasis. To our knowledge, this is the first case of APRT deficiency in New Zealand and Australia. A prompt diagnosis was made ensuring appropriate treatment and favourable prognosis for long-term renal function and stone management. However, this case highlights the risk that misdiagnosing stones as uric acid remains an issue in this rare disorder and we aim to add to the knowledge of this condition in reporting the diagnostic pitfalls.
References
- Harambat Jrm, Bollée G, Daudon M, Ceballos-Picot In, Bensman A, Group AS. Adenine phosphoribosyltransferase deficiency in children. Pediatric Nephrology. 27(4): 571-579.
- Ceballos-Picot In, Daudon M, Harambat Jrm, Bensman A, Knebelmann B, Bollée G. 2, 8-dihydroxyadenine urolithiasis: a not so rare inborn error of purine metabolism. Nucleosides, Nucleotides and Nucleic Acids. 33(4-6): 241-252.
- Edvardsson VO, Runolfsdottir HL, Thorsteinsdottir UA, Agustsdottir IMS, Oddsdottir GS, Eiriksson F, et al. Comparison of the effect of allopurinol and febuxostat on urinary 2, 8-dihydroxyadenine excretion in patients with adenine phosphoribosyltransferase deficiency (APRTd): A clinical trial. European journal of internal medicine. 48: 75-79.
- Simmonds HA, Van Acker KJ. Adenine phosphoribosyltransferase deficiency: 2, 8-dihydroxyadenine lithiasis. Metabolic basis of inherited disease/ [edited by] John B Stanbury [et al]. 1983.
- Cloutier J, Villa L, Traxer O, Daudon M. Kidney stone analysis: Give me your stone, I will tell you who you are! World journal of urology. 33(2): 157-169.
- Ceballos-Picot I, Perignon JL, Hamet M, Daudon M, Kamoun P. 2, 8-Dihydroxyadenine urolithiasis, an underdiagnosed disease. Lancet (London, England). 1992; 339(8800): 1050.
- Barratt TM, Simmonds HA, Cameron JS, Potter CF, Rose GA, Arkell DG, et al. Complete deficiency of adenine phosphoribosyltransferase: a third case presenting as renal stones in a young child. Archives of disease in childhood. 1979; 54(1): 25-31.
- Nozue H, Kamoda T, Saitoh H, Ichikawa K, Taniguchi A. A Japanese boy with adenine phosphoribosyltransferase (APRT) deficiency caused by compound heterozygosity including a novel missense mutation in APRT gene. Acta Paediatrica.100(12): e285-e8.
- Oyake C, Ikeda H, Fuyama M, Watanabe T, Isoyama K. Minimum allopurinol dose for adenine phosphoribosyl transferase deficiency. Pediatrics International. 59(10):1097-1098.
- Le Chong S, Ng YH. Obstructive uropathy and severe acute kidney injury from renal calculi due to adenine phosphoribosyltransferase deficiency. World Journal of Pediatrics.12(2): 243-245.
- Cao AE, Rodriguez TA, Sala LL, Castaneda SM, Hernando HAM, Montoya CR, et al. E36 Clinical presentation of unusual purine urolithiasis: hereditary xanthinuria and adenine phosphoribosyltransferase deficiency. European Urology Supplements. 14(4): 43.
- Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: 2, 8-Dihydroxyadeninuria. American Journal of Kidney Diseases. 69(3): e15-e6.
- Bollée G, Harambat Jrm, Bensman A, Knebelmann B, Daudon M, Ceballos-Picot In. Adenine phosphoribosyltransferase deficiency. Clinical Journal of the American Society of Nephrology. 7(9): 1521-1527.
- Rare Kidney Stone Consortium Patient Registry (RKSC). Mayo Clinic, MN: National Library of Medicine; 2008.
- Prospective Research Rare Kidney Stones (ProRKS). Mayo Clinic, MN: National Library of Medicine; 2016 [updated 2016; cited 20/09/2019]; Available from: https://clinicaltrials.gov/ct2/show/NCT02780297.

