Checkpoint Inhibitor Induced versus Paraneoplastic Vasculitis
Jacob Zaslavsky*, Colton Smith, Biren Saraiya, Sumi Thomas, Nina Ramessar
Department of Medicine, Rutgers Robert Wood Johnson Medical School, USA
Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, USA
Assistant Professor of Medicine. Rutgers Cancer Institute of New Jersey, USA
Department of Pathology and Laboratory Medicine. Rut Rutgers Robert Wood Johnson Medical School, USA
Department of Medicine, Rheumatology. Rutgers Robert Wood Johnson Medical School, USA
Received Date: 24/02/2021; Published Date: 10/03/2021
*Corresponding author: Jacob Zaslavsky, DO. Resident, Department of Medicine. Rutgers Robert Wood Johnson Medical School, Address: 125 Paterson Street, Suite 7302. New Brunswick, NJ 08901. Email address: jz780@rwjms.rutgers.edu. Phone: 201-956-2260
Abstract
Immune Checkpoint Inhibitors (ICIs) are relatively novel antineoplastic agents utilized in various malignancies, including metastatic melanoma, non-small cell lung cancer, urothelial carcinoma, among others. Currently available agents in this class include ipilimumab, nivolumab, pembrolizumab, avelumab, durvalumab, and atezolizumab. While these medications are known for their efficacy, they are unfortunately also associated with a variety of immune-related Adverse Events (irAEs), including rheumatologic and autoimmune side effects, due to activation of the body’s immune response. On the other hand, malignancy itself can often cause a paraneoplastic reaction, thus making the exact etiology of an inflammatory phenomenon difficult to ascertain. In this case, we report on a 63-year-old male diagnosed with Stage pT2 muscle invasive bladder cancer, who after receiving 3 cycles of Nivolumab, a PD-1 receptor inhibitor, was noted to have extensive non-granulomatous vasculitis of the surrounding small and medium vessels within and around the bladder, ureters, and prostate. This case helps to recognize the clear association of rheumatologic processes such as vasculitis with both malignancy and treatment for malignancy, particularly immunotherapy.
Keywords: Nivolumab; Immune check point inhibitor; Paraneoplastic; Vasculitis; Bladder cancer
Introduction
Immune Checkpoint Inhibitors (ICIs) are relatively novel antineoplastic agents utilized in various malignancies, including metastatic melanoma, non-small cell lung cancer, urothelial carcinoma, among others [1-3]. Currently available agents in this class include ipilimumab, nivolumab, pembrolizumab, and atezolizumab [4,5]. While these medications are known for their efficacy, they are unfortunately also associated with a variety of immune-related Adverse Events (irAEs), including rheumatologic and autoimmune side effects, due to activation of the body’s immune response [6,7]. This case discusses a 64-year-old gentleman with history of invasive bladder cancer treated with Nivolumab and bladder resection, who was found to exhibit small and medium vessel vasculitis noted on his resected bladder, ureters, and lymph node biopsies. This patient received extensive workup to rule out a systemic autoimmune process, which was ultimately negative. The vasculitic phenomenon was localized to the surrounding region of his malignancy, therefore it is uncertain whether the process was an adverse effect of Nivolumab therapy or a paraneoplastic effect of the cancer itself. Although there remains ambiguity in this facet, the association is clear that both cancer and immunotherapy used to treat it can result in inflammatory reactions that can have significant clinical manifestations.
Case Report
A 63-year-old Caucasian male presented initially to his primary care physician after experiencing recurrent gross hematuria for 2 months. His past medical history was significant for anxiety, osteoarthritis, benign prostatic hyperplasia, hypertension and uncomplicated renal calculi.
The patient was evaluated by Urology, and a CT scan of the abdomen/pelvis with contrast demonstrated moderate to severe left hydroureteronephrosis down to the level of the urinary bladder. At the time of cystoscopy, a tumor was found, and involved the bladder floor/left ureteral orifice. This necessitated resection with a left ureteral stent placement. The stent currently is maintained. The pathology from the cystoscopy resection was consistent with a high-grade urothelial carcinoma invasive into the muscularis propria.
The patient was evaluated by Urological Oncology and deemed appropriate for surgical resection. He was referred to medical oncology for consideration of neoadjuvant chemotherapy. He was not a cisplatin candidate and was offered a trial of neoadjuvant immunotherapy and randomized to Nivolumab for 3 cycles in pre-op setting. He received 3 cycles of Nivolumab treatment, and then proceeded to have a radical cystectomy/prostatectomy with extensive lysis of adhesions, bilateral pelvic lymph node dissection, and ileal conduit urinary diversion.
The tissue biopsy revealed invasive high grade urothelial carcinoma in the left bladder wall with tumor invasion into the muscularis propria but not beyond it. The prostate, seminal vesicles and lymph nodes were negative for tumor involvement. Interestingly, extensive non-granulomatous vasculitis involving small and medium sized arteries within and around the bladder, ureters and prostate were seen (Figure 1, 2).
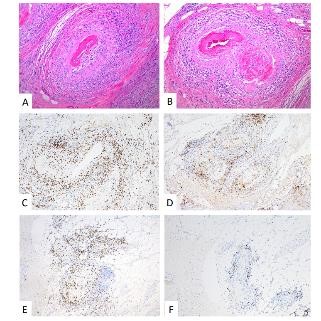
Figure 1: Prostate – H&E (A, B): Transmural lymphoplasmacytic vasculitis involving small- to medium-sized arteries and arterioles with extensive fibrinoid necrosis; IHC: Predominantly CD3+ T-cell population (C) with mixed CD4+ helper T-cells (D) and CD8+ cytotoxic T-cells (E); CD20+ B-cells (F) are not a prominent component of the infiltrate.
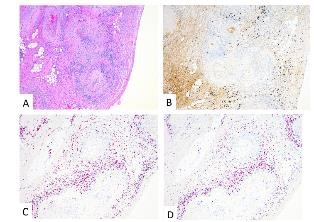
Figure 2: Ureters – H&E (A); IHC: IgG4 (B); In Situ Hybridization: Plasma cells characterized as a mixed population of kappa (C) and lambda (D) light chains with only scattered IgG4 positivity.
Microscopy demonstrated a small to medium vessel vasculitis involving arteries and arterioles. The pattern of inflammation was that of extensive fibrinoid necrosis with a predominantly lymphoplasmacytic infiltrate involving the transmural vessel wall; rare areas of acute inflammation within the vessel wall were seen associated with fibrinoid necrosis. Intimal hyperplasia, foam cell arteriopathy, and vascular wall scarring were also noted, reflecting chronicity within this lesion. Extensive immunophenotypic characterization of the inflammatory infiltrate was performed. The lymphocytes were predominantly CD3 positive T-cells with a mixed CD4 positive helper T-cell and CD8 positive cytotoxic T-cell population. The plasma cells were of mixed kappa and lambda light chains on in situ hybridization with only scattered IgG4 positive cells within the vessel adventitia; the infiltrate was not considered IgG4 dominant. CD20 positive B-cells were not a prominent component of the inflammatory infiltrate. Scattered CD68 positive macrophages were present within the infiltrate but the pattern of inflammation was not considered to be granulomatous. In summary, a necrotizing, predominantly lymphoplasmacytic vasculitis was present in acute and healing phases.
The patient was evaluated by Rheumatology within 1 week of the pathology findings, and found to be very cachexic, with no complaints of fever, rash, sinusitis, joint pain, muscle pain, limb weakness, hemoptysis, or epistaxis. Besides lethargy and overall generalized weakness, he had abdominal pain and tenderness related to the midline surgical scar and the stent placement, but no other focal features. His Anti-Nuclear Antibodies (ANA) were found to be negative, as were his Antineutrophil Cytoplasmic Antibody (ANCA) levels. He did have an elevated sedimentation rate of 46mm/Hr and a C- reactive protein of 94.2 mg/dl, but this was during the post-operative period. During follow up, he revealed a history of recurrent migraines with tinnitus and was seen by neuro-ophthalmology, but no vasculitic process was found, and this was deemed to be a chronic unrelated condition.
The patient was subsequently admitted for dehydration and for recurrent gastric ulcerations and candidiasis, which were aggressively managed. A biopsy of these ulcerations did not reveal a new vasculitic component, but rather chronic gastritis and esophagitis. A Computed Tomography Angiogram (CTA) of his chest/abdomen and pelvis was subsequently performed, and this was noted to be negative for medium or other large vessel vasculitis, with positive findings of mild aortoiliofemoral atherosclerotic disease and mild focal stenosis of the proximal SMA. There was no associated lymphadenopathy and no aneurysmal dilatations.
On re-evaluation by medical oncology approximately 1 month later, the patient was noted to have gained weight and was no longer anorexic. The patient was ultimately restarted on Nivolumab as he remained without systemic autoimmune disease, and to date has been tolerating therapy well without other manifestations of vasculitis noted.
Discussion
Immune checkpoint inhibitors are becoming increasingly utilized in clinical medicine, particularly in the field of oncology. In basic terms, the mechanism of these medications involves blocking Cytotoxic T Lymphocyte-Associated protein 4 (CTLA-4) or programmed cell death protein 1 pathways, including programmed cell death protein 1 (PD-1) and its associated ligand (PD-L1). This causes immune dysregulation, stimulating the body’s own immune system to develop an immunologic response against cancer cells [4,5,8,9]. In regards to the programmed cell death protein 1 pathway, the binding of PD-L1 to the PD-1 receptor expressed on T-cells decreases T-cell survival, avoiding an autoimmune driven reaction, and thus maintaining tolerance to self-antigens [10]. The relatively recent recognition that cancer cells often exhibit vast expression of PD-L1 has helped to explain their ability to evade T-cell attack; thus, blocking this pathway through the use of checkpoint inhibitors leads to an exaggerated immune response which allows the body’s T-cells to form an immunologic response against tumor cells [11]. The CTLA-4 pathway results in a purposeful immune dysregulation in a similar fashion [4,8].
There have been over 200 reports of rheumatologic immune-related adverse events associated with ICIs, involving diagnoses such as rheumatoid arthritis, psoriatic arthritis, polymyalgia rheumatica, spondylarthritis, and vasculitis [5], with the prevalence estimated to be around 1-10% [12]. In particular, vasculitis due to ICIs is an uncommon adverse effect, ranging from single organ involvement to systemic involvement of small, medium, and large vessels [5,6,13,14]. A systematic review by Daxini et al yielded 20 case reports of ICI-induced vasculitis, in which the predominant reported types of vasculitis were large vessel, such as GCA and isolated aortitis, as well as vasculitis of the CNS, including primary angiitis of the CNS and isolated vasculitis of the PNS [6]. Other cases included Granulomatosis with Polyangiitis (GPA), vasculitic neuropathy, cryoglobulinemic vasculitis, and digital vasculitis. Case reports have been described of single organ involvement of vasculitis in setting of ICI use, including vasculitis involving the testicles, retina, uterus, and brain[6,15-18]. A large-scale observational study by Salem et al, which reported on 31,321 adverse events associated with ICI use, confirmed 82 vasculitis cases [19]. The pathophysiology for these adverse effects is currently unknown, but there are several suggested explanations, such as increasing levels of pre-existing autoantibodies, enhancing T cell activity against self-antigens, in addition to stimulating cytokine release and complement activation [5,20]. In GCA, it is theorized that there is a lack of PD-L1 expression in vessel wall immune cells, thus leading to unopposed T cell activation and promoting inflammation; similarly, checkpoint inhibitor therapy induced vasculitis may occur via a similar mechanism via the obstruction of the PD-1/PD-L1 pathway [5,21].
A grading system instituted by The American Society of Clinical Oncology rates adverse effects attributed to immunologic agents, ranging from grade 1 (mild reaction) to grade 4 (severe). While an inflammatory vasculitis would in most cases be categorized as a grade 3-4 effect, an explicit grading system has not been established specifically for vasculitis [5,22]. Typically, this requires discontinuing the medication and also considering treatment with steroids and/or immunosuppressive agents, including hydroxychloroquine, rituximab, and cyclophosphamide[5,6,12]. It is important to note that development of any immune related adverse effects can be an indication that the agent is also immunologically effective against the malignancy, and therefore careful thought must be taken prior to prematurely discontinuing the therapy [23,24]. Checkpoint inhibitors are being used more frequently in clinical oncology, and though diagnosis is difficult due to the variability of case presentations, more cases of ICI induced vasculitis will ultimately be recognized. Treatment approach must be individualized and factor in the effect on the patient’s underlying cancer [5].
On the other hand, vasculitis may also occur as a paraneoplastic phenomenon. Many cases of Paraneoplastic Vasculitis (PNV) have been described in literature, including polyarteritis nodosa, retinal vasculitis, giant cell arteritis, and leukocytoclastic vasculitis, in malignancies such as melanoma and leukemia [6,25-28]. 2.5 to 5% of patients diagnosed with vasculitis have been estimated to have a related malignancy, which may not be apparent at initial diagnosis [29]. One study by Solans-Laqué et al reviewed records of all patients diagnosed with vasculitis and solid tumors within 12 months of each other at their institution, only including patients with biopsy proven vasculitis and malignancy [29]. Among the 15 cases described, 7 cases involved patients where the diagnosis of vasculitis preceded the cancer diagnosis, 6 cases where they were diagnosed simultaneously, and 2 diagnoses of vasculitis after malignancy diagnosis. The most common vasculitis was cutaneous leukocytoclastic vasculitis (9 cases), and others were giant cell arteritis, Henoch-Schonlein purpura, and polyarteritis nodosa, while malignancies included carcinoma of urinary organs, lung, and GI tract. 13 of the patients had corresponding disease activity and treatment response between cancer and vasculitis, and in 7 cases vasculitis flare preceded tumor recurrence or progression. Although cases of PNV are rare, the phenomenon is described in various literature. Small vessels are most commonly involved; this includes Leukocytoclastic Vasculitis (LCV), the most common manifestation of PNV which can be seen in up to 50-60% of cases [1,28-33]. Other manifestations include PAN, GPA, MPA, and HSP [28]. PNV is more strongly seen in association with hematologic disorders and malignancies than with solid tumors, however both associations have been described [1,28,31,34,35].
The pathophysiology of this phenomenon is not well understood, but several theories have been postulated. One explanation is that the increased cellular turnover from cancer cells leads to the development of autoantibodies that infiltrate vasculature. There may also be increased release of tumor cytokines and angiogenic factors, causing endothelial damage, vascular permeability and inflammation[28,34,36,37]. Other possible mechanisms involve immune complex deposition as well as direct vascular damage from tumor cells[33,38]. Management of paraneoplastic vasculitis expectedly involves treating the underlying malignancy. Effective treatment of cancer is typically correlated with a clinical improvement in the associated vasculitis[29,39]. Similarly to treatment for immunotherapy induced vasculitis, glucocorticoids and/or other immunosuppressive agents can be used to suppress underlying inflammation in certain cases[29].
Conclusion
In patients treated with a checkpoint inhibitor who are subsequently recognized to exhibit a vasculitis, it is difficult to determine if the etiology is an adverse effect of pharmacotherapy or a pre-existing association with the malignancy. Certainly, the timing of the manifestation gives insight into its etiology, whether due to ICI or paraneoplastic. Further, if the vasculitis resolves with discontinuation of the ICI, then it can likely be thought to be due to an adverse effect; if vasculitis precedes ICI therapy or persists despite discontinuation of the therapy, it may more likely be paraneoplastic. Vasculitis specifically localized to areas adjacent to tumor, such as in this case, can be either due to paraneoplastic effect or due to adverse effect of immunotherapy, and a specific diagnosis in his regard based on pathology is not feasible. The development of any immune related adverse effects can be an indication that the therapy is immunologically effective against the malignancy, and therefore careful thought must be taken prior to prematurely discontinuing therapy. If patients remain otherwise asymptomatic and without systemic manifestations of autoimmune disease, it is certainly reasonable to resume immunotherapy and monitor for signs of subsequent adverse effects, particularly when the tumor has been surgically addressed. In any case, a high degree of clinical suspicion must be maintained in order to effectively diagnose and treat patients with vasculitis and underlying malignancy.
References
- Boland P, Heath J, Sandigursky S. Immune checkpoint inhibitors and vasculitis. Current opinion in rheumatology, 2020; 32(1): p. 53-56.
- Hodi FS, O'Day SJ, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine, 2010; 363(8): p. 711-723.
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. Journal of clinical oncology, 2015; 33(17): p. 1974-1982.
- Sharon E. Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chinese journal of cancer, 2014. 33(9): p. 434-444.
- Crout TM LD, Kishore S, Majithia V. Systemic Vasculitis Associated with Immune Check Point Inhibition: Analysis and Review. Curr Rheumatol Rep, 2019. 21(6): p. 28.
- Rosenberg SA, Yang JC, Restifo NP, Cancer immunotherapy: moving beyond current vaccines. Nature medicine, 2004. 10(9): p. 909-915.
- Yang Y, Cancer immunotherapy: harnessing the immune system to battle cancer. The Journal of clinical investigation, 2015; 125(9): p. 3335-3337.
- Lindner AK, Gruenbacher G, Schachtner G, et al. Rare, but Severe: Vasculitis and Checkpoint Inhibitors. European urology focus, 2020; 6(3): p. 609-612.
- McKay RR, Bossé D, Xie W, et al. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer immunology research, 2018; 6(7): p. 758-765.
- Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clinical rheumatology, 2018; 37(9): p. 2579-2584.
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PloS one, 2016; 11(7).
- Richter MD, Crowson C, Kottschade LA, et al. Rheumatic Syndromes Associated with Immune Checkpoint Inhibitors: A Single-Center Cohort of Sixty-One Patients. Arthritis & Rheumatology, 2019; 71(3): p. 468-475.
- Gallan AJ, Alexander E, Reid P, et al. Renal Vasculitis and Pauci-immune Glomerulonephritis Associated with Immune Checkpoint Inhibitors. American journal of kidney diseases: the official journal of the National Kidney Foundation, 2019; 74(6): p. 853-856.
- Cappelli LC, Gutierrez AK, Bingham CO, et al. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis care & research, 2017; 69(11): p. 1751-1763.
- Weiner R, Hanson B, Rehman J, et al. Isolated testicular vasculitis due to immune checkpoint inhibitor. European journal of rheumatology, 2019; 7(1): p. 35-36.
- Manusow JS, Khoja L, Pesin N, et al. Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. Journal for immunotherapy of cancer, 2014; 2(1): p. 41.
- Minor DR, Bunker SR, Doyle J, Lymphocytic vasculitis of the uterus in a patient with melanoma receiving ipilimumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 2013; 31(20): p. e356.
- Läubli H, Hench J, Stanczak M, et al. Cerebral vasculitis mimicking intracranial metastatic progression of lung cancer during PD-1 blockade. Journal for immunotherapy of cancer, 2017; 5: p. 46.
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. The Lancet, 2018; 19(12): p. 1579-1589.
- Postow MA, Sidlow R, Hellman MD, et al. Immune-related adverse events associated with immune checkpoint blockade. New England Journal of Medicine, 2018; 378: p. 158-168.
- Watanabe R, Zhang H, Berry G, et al. Immune checkpoint dysfunction in large and medium vessel vasculitis. American Journal of Physiology: Heart and Circulatory Physiology, 2017; 312(5): p. 1052-1059.
- Brahmer JR, Lacchetti C, Schneider BJ, et al, Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 2018; 36(17): p. 1714-1768.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer, 2012. 12(4): p. 252-264.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA oncology, 2016; 2(10): p. 1346-1353.
- Veitch D, Tsai T, Watson S, Joshua F. Paraneoplastic polyarteritis nodosa with cerebral masses: case report and literature review. International journal of rheumatic diseases, 2014; 17(7): p. 805-809.
- Anastasakis A, Dick AD, Damato EM, Spry PG, Majid MA. Cancer-associated retinopathy presenting as retinal vasculitis with a negative ERG suggestive of on-bipolar cell pathway dysfunction. Documenta ophthalmologica. Advances in ophthalmology, 2011; 123(1): p. 59-63.
- Okada M, Suzuki K, Hidaka T, Shinohara T, Takada K, Nakajima M, Nakanishi T, Ohsuzu F. Polyarteritis associated with hypopharyngeal carcinoma. Internal medicine (Tokyo, Japan), 2002; 41(10): p. 892-895.
- Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis and rheumatism, 2007; 57(8): p. 1473-1480.
- Solans-Laqué R, Bosch-Gil JA, Pérez-Bocanegra C, Selva-O'Callaghan A, Simeón-Aznar CP, Vilardell-Tarres M. Paraneoplastic vasculitis in patients with solid tumors: report of 15 cases. The Journal of rheumatology, 2008; 35(2): p. 294-304.
- Sánchez NB, Canedo IF, García-Patos PE, de Unamuno Pérez P, Benito AV, Pascual AM. Paraneoplastic vasculitis associated with multiple myeloma. Journal of the European Academy of Dermatology and Venereology: JEADV, 2004; 18(6): p. 731-735.
- Hayem G, Gomez MJ, Grossin M, Meyer O, Kahn MF, Systemic vasculitis and epithelioma. A report of three cases with a literature review. Revue du rhumatisme (English ed.), 1997; 64(12): p. 816-824.
- Ungprasert P, Sanguankeo A, Upala S, Knight EL. Risk of malignancy in patients with giant cell arteritis and polymyalgia rheumatica: a systematic review and meta-analysis. Seminars in arthritis and rheumatism, 2014; 44(3): p. 366-370.
- Fam AG. Paraneoplastic rheumatic syndromes. Best Practice & Research Clinical Rheumatology, 2000; 14(3): p. 515-533.
- Park HJ, Ranganathan P. Neoplastic and paraneoplastic vasculitis, vasculopathy, and hypercoagulability. Rheumatic diseases clinics of North America, 2011; 37(4): p. 593-606.
- Racanelli V, Prete M, Minoia C, Favoino E, Perosa F. Rheumatic disorders as paraneoplastic syndromes. Autoimmunity reviews, 2008; 7(5): p. 352-358.
- García-Porrúa C, González-Gay MA, Cutaneous vasculitis as a paraneoplastic syndrome in adults. Arthritis and rheumatism, 1998; 41(6): p. 1133-1135.
- Fortin PR. Vasculitides associated with malignancy. Curr Opin Rheumatol, 1996; 8(1): p. 30-33.
- Buggiani G, Krysenka A, Grazzini M, Vašků V, Hercogová J, Lotti T. Paraneoplastic vasculitis and paraneoplastic vascular syndromes. Dermatologic therapy, 2010; 23(6): p. 597-605.
- Azar L, Khasnis A. Paraneoplastic rheumatologic syndromes. Current opinion in rheumatology, 2013; 25(1): p. 44-49.

