Isolated Oral Pemphigus Vulgaris
Richmond Ronald Gomes*, Inteha Afrin
Associate Professor, Medicine, Ad-Din Women’s Medical College Hospital, Bangladesh
Received Date: 25/01/2021; Published Date: 11/02/2021
*Corresponding author: Associate Professor, Medicine, Ad-Din Women’s Medical College Hospital, Dhaka, Bangladesh, Intern, Medicine, Ad-Din Women’s Medical College Hospital, Dhaka, Bangladesh. Email: rrichi.dmc.k56@gmail.com, Orchid ID: 0000000225117972.
Abstract
Pemphigus Vulgaris (PV), a rare autoimmune mucocutaneous intraepithelial blistering disease has been reported with incidence of 0.1-0.5 cases per 100,000 individuals worldwide per year. The etiology of PV is uncertain but is supposed to be mediated by circulating immunoglobulin G (IgG) autoantibodies against the desmosomal cadherins, desmoglein 1 and 3. Biopsy, light microscopic examination, and additional adjuvant tests, such as immunofluorescence studies can be used to establish the diagnosis. In most cases (70-90%), the first signs of disease appear on the oral mucosa but most patients with oral lesions are initially misdiagnosed and treated improperly for months or years. If these patients are misdiagnosed or left untreated, PV may be fatal with a mortality rate ranging from 60% to 90%. We report the case of a 39-years old woman with a 4-month history of oral ulcerations. The patient reported that the lesions caused considerable discomfort and affected her normal oral function. On intraoral examination, ulcers were observed on both cheeks and ventral surface of tongue. No skin lesions were seen on extra oral examination. A diagnosis of PV was made after evaluating the biopsy samples. The main complication of PV is the reduced quality of life related to soreness or pain, particularly in ulcerative/erosive lesions. Therefore, here we are trying to discuss basics of diagnosing and treating PV with oral lesions with the help of a case report.
Keywords: Autoimmune blistering diseases; Desmoglein; Pemphigus vulgaris; Cadherins; Direct and indirect immunofluorescence
Introduction
Pemphigus represents a group of rare chronic inflammatory autoimmune mucocutaneous blistering disorders. The word pemphigus originates from Greek pemphix, which translates as blister or bubble. Pemphigus can be classified into five major groups: Pemphigus Vulgaris (PV), pemphigus foliaceus, Paraneoplastic Pemphigus (PNP), drug-induced pemphigus and immunoglobulin A (IgA) pemphigus. Oral lesions have been associated with only PV and PNP. Pemphigus Vulgaris is the most common form of pemphigus, accounting for over 80% of cases. Pemphigus vulgaris is a rare disorder, with a reported incidence of 0.1-0.5 cases per 100,000 individuals worldwide per year. It occurs primarily in adults during 5th or 6th decade of life with a male female ratio 1:2 [1].
The disease is mediated by circulating immunoglobulin G (IgG) autoantibodies against the desmosomal cadherins, desmoglein 1 and 3(the PV antigen), a transmembrane glycoprotein which mediates cell adhesion [2]. Although the exact mechanism is unclear, autoantibodies theoretically produce an allosteric change in the desmoglein, impairing its adhesive abilities, and increase active plasmin in the area, producing cell degradation and acantholysis [2,3]. Complement may be actively involved in this process3.Histopathology reveals a loss of cell-cell adhesion (acantholysis) in the supralaminar layer of the epithelium, and Direct Immunofluorescence (DIF) of perilesional skin reveals intercellular deposition of IgG +/- C3. As antibodies often correlate with disease activity, Indirect Immunofluorescence (IIF), immunoblots, and Enzyme-Linked Immunosorbent Assays (ELISA) are commonly used to quantify circulating antibody level [4].
To make the diagnosis of pemphigus vulgaris, the clinician needs to perform a biopsy of the lesional tissue [5]. If left untreated, PV is frequently fatal with a mortality rate ranging from 60% to 90% [6]. Characteristically, PV lesions usually start affecting the oral mucosa, followed by the appearance of skin lesions months later [7,8]. Lesions may occur anywhere on the oral mucosa, but the buccal mucosa is the most commonly affected site followed by involvement of the palatal, lingual and labial mucosa. Gingiva is the least commonly affected site. Most patients are initially misdiagnosed and improperly treated for many months or even years. In the majority of patients, it is sometimes difficult to diagnose when only mucosal involvement is present. If oral pemphigus vulgaris can be recognized in its early stages, treatment may be initiated to prevent the progression of the disease to skin involvement. The purpose of the current study was to present the management of a patient with a history of painful oral lesion, who was finally diagnosed as having PV.
Case Report
A 39-years-old Bangladeshi lady reported to the medicine department with the complaints of recurrent painful ulcers in the mouth and difficulty in swallowing solid food and liquids for last 4 months. There was sore throat at first followed by ulcers all over mouth, which healed incompletely in 2½ months after medication by local practitioner, but the ulcers recurred after stoppage of medication. History revealed that the ulcerations started initially as blisters and were associated with pain that was aggravated on chewing food. The ulcerations caused considerable discomfort, affecting his normal oral functions. The patient had noticed ulcers of the mouth which bled on brushing, and increased salivation in the morning was reported. The patient did not report of skin lesions or involvement of other mucosal sites. She also denied for any genital or anal ulcer, joint pain, photosensitive rash, fetal loss. A review of medical and family history was noncontributory. There was no history of long-term treatment for any chronic illness or continuous drug intake. The patient had poor oral hygiene with adverse habit of taking betel quid for 10 years. On general examination, the patient was moderately built and no signs of anemia were present. Submandibular lymph nodes were enlarged, palpable and tender bilaterally. Intra-oral examination revealed ulcerative lesions present on bilateral buccal mucosa along the line of occlusion extending from retrocommisural areas to the retromolar trigone posteriorly (Figure 1 and 2). Lesions extended superiorly from the line of occlusion and were irregular in shape covered by pseudo membrane with erythematous surrounding. On manipulation, bleeding was present. Lesions were also present on ventral surface of the tongue on left side (Figure 3). There were diffuse areas of erosions covered by pseudo membrane at some sites. Nichols’s sign showed a positive reaction. Generalized teeth attrition and gingival inflammation with bleeding on probing were present.
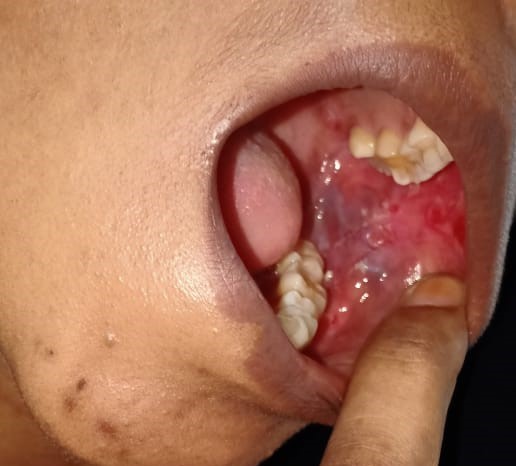
Figure 1
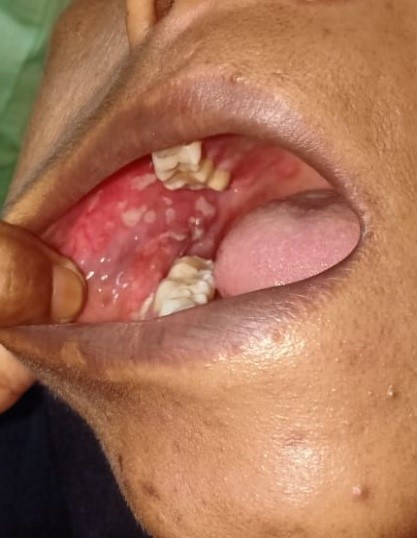
Figure 2
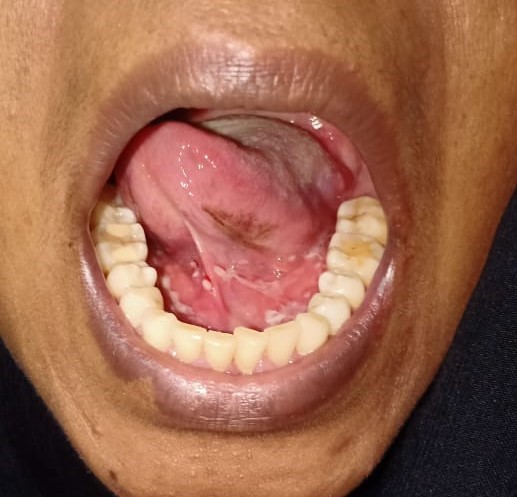
Figure 3
Ulcerative lesions on bilateral buccal mucosa along the line of occlusion extending from retrocommisural areas to the retromolar trigone posteriorly, irregular in shape covered by pseudo membrane with erythematous surrounding. Figure 3: Lesions on ventral surface of the tongue on left side.
The clinical presentation of chronic multiple oral ulcers, flaccid bullae and positive Nikolysky sign in this case led to provisional diagnosis of vesicular-bullous lesion affecting the oral cavity. Differential diagnosis included pemphigus vulgaris, mucous membrane pemphigoid, bullous lichen planus, para neoplastic pemphigus, chronic ulcerative stomatitis, recurrent herpes lesions in immunocompromised patients and erythema multiforme.
Routine hematological and biochemical investigations including liver function tests, renal function tests were within normal limits. Serology for hepatitis B, Hepatitis C, HIV and syphilis were negative. Vasculitis screening with ANA, ANCA were not conclusive. Incisional biopsy was performed from peri lesional site of the right buccal mucosa. Histopathological examination revealed hyperkeratotic and acanthotic squamous epithelium. The surface is ulcerated at places with subproposal bullae containing acanthotic cells (Figure 4). Based on the histopathological findings, a final diagnosis of pemphigus vulgaris was made.
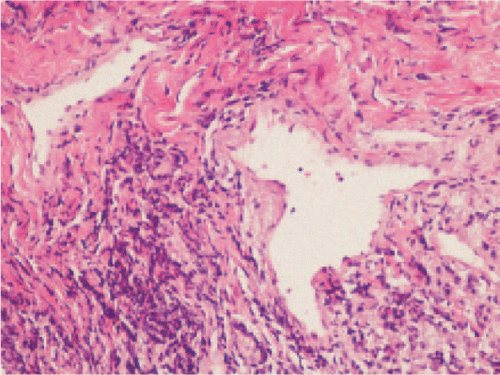
Figure 4: Histopathologic examination of specimens from the oral sample of pemphigus vulgaris shows acantholysis in the lower spinous cell layers. Basal layer cells are attached to the connective tissue and subproposal cleft are seen at the tips of the epithelial rete ridges
The treatment plan comprised oral prednisolone 60 mg/day along with calcium and vitamin D supplements and analgesic. Topical analgesic mouthwash and 0.1% Triamcinolone acetonide ointment were also prescribed to the patient. On the first follow-up after 3 weeks, the patient had 50% reduction in symptoms with partial healing of lesions, erythema and inflammation in relation to ulcers had reduced (Figure 5 and Figure 6). Over the past 6 months, prednisolone was gradually tapered down as there was complete regression of the lesions. The patient is still in follow-up and is doing well.
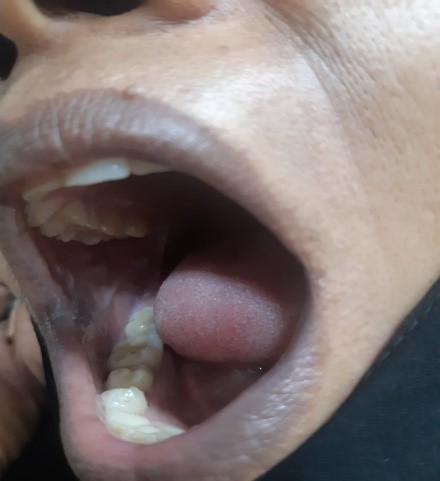
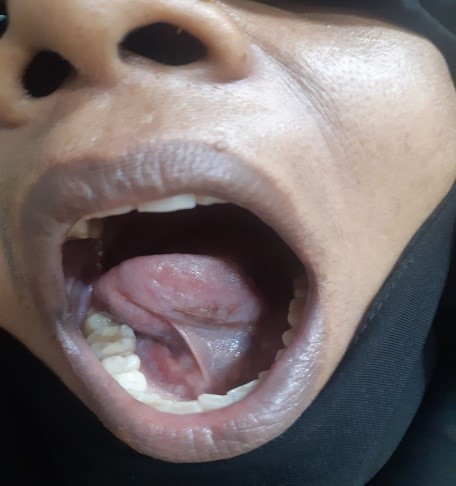
Figure 4 and Figure 5: Partial healing of oral lesion with reduction of erythema and inflammation.
Pemphigus vulgaris is a rare cause of chronic ulceration of the oral mucosa. The mouth may be the only site of involvement for a year or so. In this case, early diagnosis and lower doses of medication for shorter period of time was could control the disease.
Discussion
Pemphigus is defined as a group of life-threatening blistering disorder of skin and mucous membrane characterized by acantholysis (loss of keratinocyte to keratinocyte adhesion). The process of acantholysis is induced by circulatory autoantibodies to intercellular adhesion molecules [9]. There are five major categories of pemphigus: PV, pemphigus foliaceus, PNP, drug-induced pemphigus and IgA pemphigus. Each form of this disease has antibodies directed against different target cell surface antigen, resulting in a lesion forming in different layers of the epithelium [10]. The oral mucosa is the initial site of involvement in 70%-90% of the cases before involvement of the skin and other mucosal sites [1]. Nasal, conjunctival, pharyngeal, laryngeal, esophageal and genital mucosa are the other mucosal sites that may be affected.
The etiology of PV is uncertain as is with other auto-immune diseases [17,18]. In some cases, it is found to have a strong genetic basis, as it has been reported in certain racial groups, for example, the Ashkenazi Jews and those of Mediterranean descent. Strong association between certain HLA class II alleles has also been demonstrated. Other initiating factors include certain foods, infection, neoplasms, and drugs. The drugs commonly implicated are those in the thiol group, in particular captopril, penicillamine, and others such as rifampicin6. PV is believed to occur as a result of autoantibodies directed against the components of epithelial desmosomes. The desmosomes consist of two membrane-bound adhesion molecules, Dsg [11] and desmocollin, which are linked to the cytoskeleton through intracellular plakoglobin, plakophilin and des mop akin [12]. Dsg consists of two subtypes, Dsg-1 and Dsg-3, which are expressed variably in the skin and oral mucosa. Dsg-3 is expressed in the basal layers of the epidermis while the superficial epidermal cells predominantly express Dsg-1. In the oral mucosa, Dsg-3 expression predominates over Dsg-1. Thus, the oral epithelial integrity is exclusively dependent on Dsg-3, which is targeted by the autoantibodies in cases of PV affecting the oral cavity [6].
The binding of Pemphigus Vulgaris antibody activates protease, whereas more recent evidence supports the theory that the Pemphigus vulgaris antibodies directly block the adhesion function of the desmoglein [13,14,15]. The separation of cells called acantholysis takes place in the lower layers of stratum spinosum, which results in the formation of supralaminar bulla. The bulla increasingly involves a larger area of epithelium, resulting in loss of large area of skin and mucosa.
The classical lesion of pemphigus is a thin walled bulla that are very fragile and rupture easily, giving rise to painful and hemorrhagic erosions arising on otherwise normal skin or mucosa. A characteristic sign of the disease may be obtained by the application of pressure to intact bullae. In a patient with Pemphigus Vulgaris, bullae enlarge by extension to an apparently normal surface. Another characteristic sign of the disease is the pressure to apparently normal area resulting in the formation of a new lesion. This phenomenon, called Nikolysky sign, results from the upper layer of the skin pulling away from the basal layer. Nikolysky sign is also found to be positive in toxic epidermal necrolysis, scalded skin syndrome (both of which are acute conditions) and mucous membrane pemphigoid [16].
Most patients with oral lesions could be initially misdiagnosed, usually as aphthous stomatitis, gingivostomatitis, erythema multiforme, erosive lichen planus, or oral candidiasis, and may be improperly treated for months or years. Other differential diagnoses include dermatitis herpetiformis and cicatricial pemphigoid1. If proper history is taken, the clinician should be able to distinguish the lesions of pemphigus from those caused by acute viral infections like herpes and erythema multiforme. Immunosuppressed patients present with recurrent herpetic simplex infections in the form of atypical ulcers, which may last several weeks or months if undiagnosed and untreated. Moreover, the presence of Tzank cells may complicate the diagnosis. Since in the present case, the patient did not give history of immunocompromise like chemotherapy, organ transplant or acquired immune deficiency syndrome, recurrent herpes simplex infection could be safely ruled out.
Differential diagnosis of Pemphigus Vulgaris can be done from other similar conditions by biopsy and direct immunofluorescence. Biopsies are best done on intact vesicles and bullae less than 24 hours old. The biopsy specimen should be taken from the advancing edge of the lesion, where the area of characteristic supralaminar acantholysis can be observed by the pathologist. Supra basilar split seen in Pemphigus Vulgaris helps distinguish this condition from sub-epithelial blistering diseases such as mucous membrane pemphigoid, bullous lichen planus and chronic ulcerative stomatitis. Indirect immunofluorescence is helpful in further distinguishing pemphigus from pemphigoid and other chronic oral lesions and is useful in following the progress of patient for pemphigus. The diagnosis is confirmed by the characteristic deposition of IgG and other C3 antibodies that bind to cell surface of perilesional skin or mucosa19,20. Indirect immunofluorescence is less sensitive than direct immunofluorescence, but may be helpful if biopsy is difficult. ELISA has been developed that can detect desmoglein 1 and 3 in serum sample of patients with Pemphigus Vulgar [20]. The presence of anemia and submandibular gland lymphadenopathy along with painful stomatitis and acantholysis on histopathological investigations in the present case led to the differential diagnosis of paraneoplastic pemphigus. Absence of underlying lymphoproliferative disorder, the reduced severity of the lesions and absence of inflammation at the dermal-epidermal junction and keratinocyte necrosis in addition to characteristic acantholysis helped rule out this condition. Also, direct immunofluorescence of para neoplastic pemphigus shows deposition of IgG and complement along the basement membrane as well as on the keratinocyte surface in an intercellular location [21].
An important aspect of patient management is early diagnosis when lower doses of medication can be used for shorter periods of time to control the disease. Dental professionals must be sufficiently familiar with clinical manifestation of pemphigus vulgaris to ensure early diagnosis and treatment, since this in turn determines the prognosis and course of the disease. Institution of early treatment could prevent serious involvement of other mucosa and cutaneous sites and fatal complications. Without proper treatment, this condition can be fatal because of loss of the epidermal barrier function, leading to loss of body fluids and secondary bacterial infection [22]. Pemphigus Vulgaris is generally managed with local and systemic corticosteroid therapy. Treatment is administered in 2 phases: a loading phase, to control the disease, and a maintenance phase, which is further divided into consolidation and treatment tapering. Local treatment consists of a paste, an ointment or a mouthwash administered alone or in conjunction with systemic treatment. Intralesional injections of corticosteroids have been used for the management of persistent lesions [23]. In cases of extensive oral lesions or involvement of other mucosa and skin, systemic corticosteroid therapy is initiated immediately. The initial of prednisone 0.5–2 mg/kg is recommended [24]. Depending on the response, the dose is gradually decreased to the minimum therapeutic dose, taken once a day in the morning to minimize side effects. When steroids are used for longer periods of time, adjuvants such as Azathioprine or Cyclophosphamide are added to the regimen to reduce the complications of long-term corticosteroid therapy. Before the advent of corticosteroid therapy, pemphigus was fatal, with a mortality rate of up to 75% in the first year. It is still a serious disorder, but the 5% to 10% mortality rate is now primarily due to the side effects of therapy [25].
Emerging therapies include intravenous immunoglobulins, plasmapheresis, immunoadsorption (IA), Extracorporeal Photochemotherapy (ECP), monoclonal antibodies such as rituximab, Tumor Necrosis Factor-Alpha (TNF-α) antagonists, viz., infliximab and etanercept, cholinergic agonists, and other experimental therapies such as desmoglein 3 peptides and KC7064. Some studies have suggested Co-enzyme Q10 as adjuvant therapy for periodontal involvement in PV [26].
If undiagnosed, PV may lead to grave consequences. The article focuses on timely diagnosis and appropriate management of a pemphigus patient. Therefore, we need to induce and maintain remission with the lowest possible doses of medication, so as to minimize the risk of serious and potentially fatal adverse effects.
Conclusion
PV is a serious disease, and if left untreated, it could lead to patient's death. Often the oral mucosa is the first involved site even before the skin and other mucosal sites are affected, so the dentist may have the opportunity to play an important role in diagnosing the disease. With early detection, PV is more easily managed.
References
- Dagistan S, Goregen M, Miloglu O, Cakur B. Oral pemphigus vulgaris: A case report with review of the literature. J Oral Sci 2008; 50: 359-362.
- European Academy of Dermatology and Venereology (EADV). Journal of the European Academy of Dermatology and Venereology 29: 405–414.
- Spindler V, Waschke J. Desmosomal cadherins and signaling: Lessons from autoimmune disease. Cell communication and Adhesion .2014; 21: 77-84.
- Prajapati V, Mydlarski PR. Advances in pemphigus therapy. Skin Therapy Lett 2008; 13: 4-7.
- Chi AC, Ravenel MC, Neville BW, Bass EB Jr. A paient with painful ulcers. J Am Dent Assoc 2006; 137:626-629.
- Robinson NA, Leo JF, Lee YS, Aw DC. Oral pemphigus vulgaris: A case report and literature update. Ann Accad Singapore 2004; 33(4): 63-68.
- Kauvsi S, Danesh pazhooh M, Farahani F, Abedini R, Lajevardi V, Chams davatchi C. Outcome of Pemphigus Vulgaris. Journal of the European Academy of Dermatology and Venereology. 2008; 22(5): 580–584.
- Endo H, Rees TD, Hallmon WW. Disease progression from mucosal to mucocutaneous involvement in a patient with desquamates gingivitis area with Pemphigus Vulgaris. Journal of Periodontology. 2008; 99(2): 368–375.
- Michael H, Carrian S. Pathogenesis, Clinical Manifestation and diagnosis of Pemphigus. 2013.
- Huntley AC. Pemphigus Vulgaris and vegetating and verrucous lesions. Case Report, Dermatol Online J. 2004: 9.
- Al-Jassar C, Bikker H, Overduin M, Chidgey M. Mechanistic basis of desmosome-targeted diseases. J Mol Biol. 2013; 425: 4006–4022.
- Damoiseaux J. Bullous skin diseases: Classical types of autoimmune diseases. Scientifica (Cairo) 2013; 45: 79-82.
- Mahoney MG, Wang Z, Rothenberger K. Explanation for the Clinical and Microscopic localization of lesion in Pemphigus foliaceous and vulgaris. J Clin Invest. 1999; 103: 461–468.
- Jensen PJ, Barad J, Morioka S. Epidermal Plasminogen activator is abnormal in cutaneous lesion. J Invert Dermatol. 1988; 90: 777.
- Stanley Cell adhesion molecule as target of autoantibodies in pemphigus and pemphigoid bullous due to defective cell adhesion. Adv Immunol. 1993; 53: 291–393.
- Urbano FL. Nikolsky's Sign in Autoimmune Skin Disorders. Hospital Physician. 2001: 23–24.
- Praveen K, Subrahmanyam D. A rare cause of Guillain-Barre syndrome. Int J Nutr Pharmacol Neurol Dis 2011; 1: 204-205.
- Parashari UC, Khanduri S, Bhadury S, Srivastava D, Saxena S. The magnetic resonance imaging "wine glass" sign of amyotrophic lateral sclerosis. Int J Nutr Pharmacol Neurol Dis 2011; 1: 206-208.
- Kancoar AJ, Deo Pemphigus in India. Indian J Dermatol Venereai leprol. 2011; 77: 439–449.
- Harman KE, Albert S, Black MM British Association of Dermatologists, author. Guideline for the Management of pemphigus Vulgaris. Br J Dermatol. 2003; 149: 926–937.
- Anhalt GJ. Paraneoplastic Pemphigus. Journal of Investigative Dermatology Symposium Proceedings. 2004; 9: 29–33.
- Amagai M. Autoimmune and infectious skin diseases that target desmogleins. Proc Jpn Acad Ser B Phys Biol Sci. 2010; 86: 524–537.
- Fellner MJ, Sapadin AN. Current therapy of pemphigus vulgaris. Mt Sinai J Med. 2001; 68(4–5): 268–278.
- Toth GG, Jonkman MF. Therapy of pemphigus. Clin Dermatol. 2001; 19(6): 761–767.
- Lever WF, Schaumburg-Lever G. Treatment of pemphigus vulgaris. Results obtained in 84 patients between 1961 and 1982. Arch Dermatol. 1984; 120(1): 44–47.
- Prashanth J, Jesudoss Prabhakaran AC. The beneficial effect of Coenzyme Q in diabetic neuropathy: An overview. Int J Nutr Pharmacol Neurol Dis. 2012; 2: 80-83.

