Pathohistological and Immunohistochemical Characteristics of Locally Advanced Papillary Serous Endometrial Carcinoma - Differential Diagnosis, Prognosis and Complex Treatment
Marinova Lena*, Malinova Doroteya and Gyuler Solakova
1Department of Radiotherapy, Oncology center, Russe, Bulgaria
2Department of General and Clinical Pathology, Medical University, Varna, Bulgaria
3 Department of Chemotherapy, Oncology center, Russe, Bulgaria
Received Date: 15/10/2020; Published Date: 16/11/2020
*Corresponding author: Marinova Lena, Department of Radiotherapy, Oncology center, Russe, Bulgaria. E-mail: rad_marinova@abv.bg
Abstract
We present a clinical case of a rarely diagnosed serous endometrial carcinoma (SEC) with infiltration of the cervix, left parametrium, right fallopian tube, with bilateral ovarian metastases and tumor cells in the peritoneal lavage. After extensive pathohistological and immunohistochemical examinatoin, it was proved to be a locally advanced serous endometrial carcinoma - pT3b N0 M0. The publication discusses the importance of immunohistochemical (IHC) differential diagnosis for determining the histogenesis of papillary serous adenocarcinoma. The main pathohistological dilemma is the determination of the histological subtype of this locally aggressive endometrial carcinoma. Another problem is the definition of the primary origin of the tumor, due to the difference between the pathohistological classification in cervical, uterine and tubo-ovarian adenocarcinomas, as well as the radically different postoperative adjuvant treatment. Incorrect differential diagnosis leads to misdefinition of the necessary complex oncological treatment.
Keywords: Endometrial Serous Carcinoma; Papillary Serous Uterine Cancer; Immunohistochemistry; Differential Diagnosis; Prognosis; Complex Treatment
Introduction
Uterine Serous Cancer (USC), although rare, is the most lethal type of uterine cancer. It has distinct molecular features and pathogenetic pathways compared with other uterine cancer types [1]. According to Bokhman’s 1983 model, Endometrial Carcinoma (EC) is broadly classified based on histopathologic features into two categories, type I and type II, which differ in incidence, prognosis, epidemiology, molecular pathology, and clinical behavior [2]. Type II tumors (nonendometrioid carcinomas), such as serous carcinoma, clear cell carcinoma, carcinosarcoma/malignant-mixed Müllerian tumor, are characterized by poorly differentiated histology and deep migration/invasion [1]. Among the most common problems encountered in practice is the distinction between primary endometrial and primary endocervical adenocarcinomas, and the determination of the tumor origin when there is synchronous, multifocal involvement of gynecologic tract sites, for example the endometrium and the ovary [3]. In this article we present a clinical case of locally advanced serous uterine cancer with difficult differential diagnosis both in terms of histogenesis and histological features, as well as in terms of its primary origin, which is extremely important for complex treatment.
Clinical Case
It concerns 51-year-old woman with pain and bloating. After three peritoneal punctures, a metastatic effusion from adenocarcinoma with probable ovarian origin was found. After CT scan of the abdomen and pelvis, bilateral ovarian formations were found. A total class II laparohysterectomy with bilateral adnexectomy, omentectomy and appendectomy was performed. Intraoperatively - In situ- Ascites- 6 liters of clear liquid. Uterus of normal size. Bilateral ovarian formations with a maximum diameter of about 9 and 8 cm. There were no enlarged iliac and paraaortic lymph nodes. Liver - smooth surface without pathological changes. There were no peritoneal metastases. Radical hysterectomy with bilaterally adnexectomy, omentectomy and lymphadenectomy of the iliac lymph nodes were performed.
Histological Result
In the uterus a polypoid tumor mass was found with complex papillary and glandular architecture, infiltrating the myometrium up to 5 mm; the gland-like spaces were without sharp luminal borders, necrotic material was present in the lumens (Figure 2B). In the isthmus (Figure 3A) and in the cervix (Figure 1, Figure 2A) the histological findings were similar - tumor, composed of merging papillary and gland-like structures was observed, infiltrating over ½ of the muscular layer and reaching the orificium externum canalis cervicalis. In the right ovary (Figure 2C) and in the right fallopian tube (Figure 3B,3C), the same tumor, composed of complex papillary and gland-like structures was discovered, papillary structures were found on the outer surface of both ovaries, as well. Among the tumor cells in the uterus, isthmus, cervical canal, and right fallopian tube, psammoma bodies were found, as well as necrotic debris in the lumens of the tumor glands. In the left ovary cysts lined by stratified collumnar epithelium were found. The left fallopian tube was with fibro-sclerotic changes and paratubal cysts. The right parameter was with preserved architecture. The left parameter was invaded by the tumor. Five right pelvic lymph nodes have reactive changes. Seven left pelvic lymph nodes have reactive changes. Omentum - with hyperemia and mesothelial proliferation. The ascites fluid analysis reports erythrocytes, mesothelial cells, tumor cells with large nuclei - single and in small complexes of 3-4 cells. It is about metastatic effusion.
Histological Diagnosis
Рapillary serous endometrial carcinoma (рТ3b N0 M0) with predominantly exophytic growth, infiltrating the myometrium up to 5 mm., with local invasion of the right fallopian tube and left parametrium. Рapillary serous endometrial carcinoma infiltration in the cervical canal with papillary and glandular architecture reaching the orificium externum canalis cervicalis with infiltration above ½ of the muscle layer. Metastases in both ovaries. Metastatic peritoneal effusion.
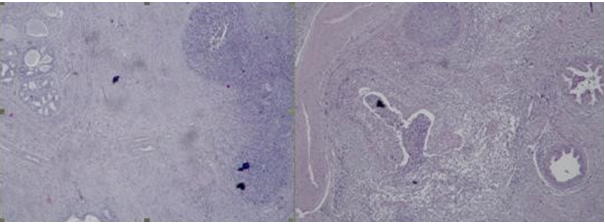
Figure 1: Photomicrography of papillary serous endometrial carcinoma in the cervical canal – Complex merging papillary and gland-like structures of tumor cells invade the cervical stroma, necrosis in the lumens is seen and psammоma bodies are present, H&E, x40.
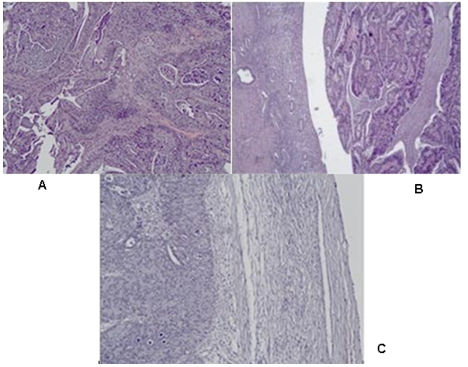
Figure 2: Photomicrography of papillary serous endometrial carcinoma: Сomplex merging papillary and gland-like structures are seen, necrotic debris in the lumens are present: A/ in in the cervical canal H&E, x100; B/ In the uterine myometrium H&E, x40; C/ In the right ovary H&E, x100.
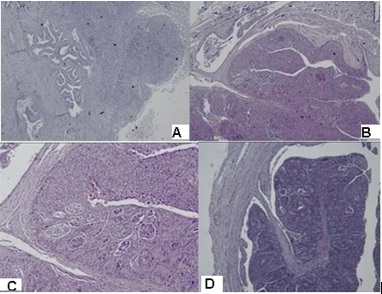
Figure 3: Photomicrography of papillary serous endometrial carcinoma: Сomplex merging papillary and gland-like spaces are seen, necrotic debris in the lumens are present. A/ isthmus, H&E x40; B/ right fallopian tube H&E, x40; C/ right fallopian tube, H&E,x100; D/ right fallopian tube, H&E x40.
Immunohistochemistry (IHC)
1/ Expression of p16 for HPV: Diffuse expression of p16 in the cervical, endometrial and ovarian tumors was reported (Figure 4).
2/ Expression of estrogen (ER) and progesterone (PR) hormonal receptors: diffuse ER expression in the cervical tumor, focal positive ER expression in the tumor in endometrial tumor and focal positive ER and PR expression in the ovarian tumor masses was reported (Figure 5).
3 / Expression of Vimentin: Diffuse expression in the tumor cells of the cervical and ovarian tumors was reported (Figure 6).
4 / Expression of EMA: positive reaction in the cervical tumor cells (Figure7).
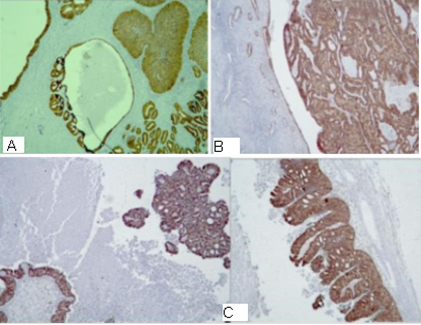
Figure 4: Photomicrography, p16 expression in the tumor cells: A/ diffuse expression in the cervical tumor ; B/ diffuse expression in the endometrial tumor ; C/ diffuse expression in the tumor in the ovarian tumors, x40.
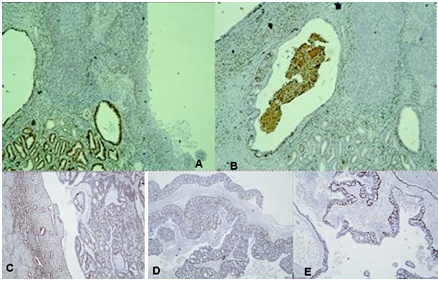
Figure 5: Photomicrography IHC - Expression of estrogen (ER) and Progesterone Receptors (PR) in the tumor cells: A/ diffuse expression of ER in the cervical tumor; B/ weak expression of PR in the cervical tumor; C/ focal positive expression of ER in the endometrial tumor; D/ and E/ focal positive expression of ER and PR in the ovarian tumors, x40.
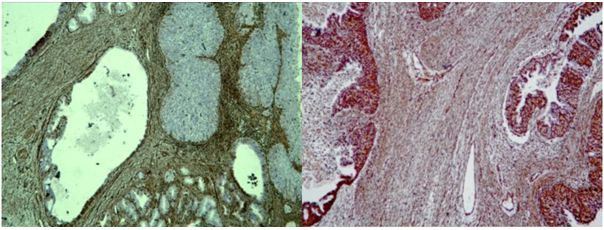
Figure 6: Photomicrography, IHC – expression of Vimentin in the tumor cells: A/ diffuse expression in the tumor in the cervical tumor; B/ diffuse expression in the ovarian tumors, x40.
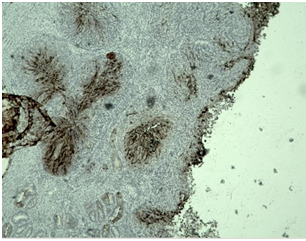
Figure 7: Photomicrography, IHC - positive EMA reaction in the tumor cells of the cervical tumor, x100.
Discussion
Uterine papillary serous carcinoma (UPSC) is an uncommon form of endometrial cancer that typically arises in postmenopausal women. UPSC is staged like other forms of endometrial carcinoma at time of surgery using the FIGO cancer staging system. In case of infiltration of serosa or adnexa and / or tumor cells into ascites or peritoneal lavage, the carcinoma is classified as stage 3A. The tumor spread in the vagina and/or parametrium (direct extension or metastases) is staged as stage 3B according to the modified FIGO classification on 1/1/2010 and “Pathologic TNM staging of carcinoma and carcinosarcoma of the corpus uteri, AJCC 8th edition and FIGO 2018 update“[4,5]. For patients who undergo surgical staging—consisting of open abdominal exploration, hysterectomy, bilateral adnexal removal, and pelvic and para-aortic lymphadenectomy in the modified FIGO classification, peritoneal lavage cytology is no longer considered in tumor staging. In the presented clinical case, despite the metastases in the ovaries, the tumor is stage 3B.
Pathohistological characteristics of serous carcinoma adenocarcinoma: Complex papillary, solid or glandular architecture; The architecture is papillary with or without appreciable fibrovascular cores; slit-like spaces; gland-like spaces may be observed (but luminal borders are not sharp as seen in endometrioid carcinoma); solid growth. Psammoma bodies may be present in up to 33% of cases. Cytoplasm is usually scant but can be abundant with eosinophilia or clearing. Nuclei are typically high grade with pleomorphism, hyperchromasia, prominent nucleoli and frequent mitotic figures (including atypical mitotic figures) [6-8]. Most type II endometrial carcinomas, especially UPSC, have a complex papillary or glandular architecture, which is similar to serous papillary ovarian carcinomas. In general, densely fibrotic papillae fronds and slit-like spaces are common [1] [Figure 2,3]. Huge, round, undifferentiated tumor cells with a high number of mitotic figures, increased nuclear-to-cytoplasmic ratios, and prominent nuclear atypia are detached and present in the blank spaces. Hobnail cells, clear cells, and polygonal cells are frequently observed. Cilia are also observed in some cases, as in papillary serous ovarian carcinomas [9,10]. Serous carcinoma cells are arranged in true papillae lined with many cells with overlapping nuclei; cells are large, better preserved and with indistinct cell borders; nuclear chromatin is coarse with large prominent nucleoli [11] (Figure 2, Figure 3).
Immunohistochemical stains in papillary serous endometrial carcinoma show that the tumor is positive for p53 (strong and diffuse expression, mutated) and p16 (strong and diffuse expression) (Figure 4), focally positive for ER (Figure 5C) and negative for PR [6]. In the presented case all tumors (in the different parts of the genital tract) demonstrated diffuse/moderate-strong p16 expression (Figure 4), with percentage of positive tumor cells ranging from 90% to 100%. In contrast, endometrial endometrioid carcinomas exhibit less diffuse and less intense expression, with percentage of positive tumor cells ranging from 10% to 90% [12]. In endometrial endometrioid carcinoma, ER and PR are strongly positive; p53 and p16 are weak and patchy [6]. Highly expressed EMA by most adenocarcinomas is associated with poor prognosis [13,14].
Differential diagnosis (DD): Non-endometrioid carcinomas, which account for about 10% of endometrial carcinomas, may pose a great array of problems in differential diagnosis, including their distinction not only from benign lesions but also from endometrioid carcinoma and various tumors that may secondarily involve the uterine corpus [15]. The complex papillary architecture of serous uterine carcinoma causes a number of diagnostic difficulties. The differential diagnosis includes Villoglandular Variant Of Endometrial Carcinoma. Papillary endometrioid or villoglandular adenocarcinoma (VGAC) is a relatively common type of well-differentiated endometrial adenocarcinoma [16,17]. VGAC is characterized by a papillary architecture with delicate fibrovascular stalks lined by cuboidal to columnar cells with minimal cellular stratification and mild nuclear pleomorphism [16]. VGAC only superficially invades the myometrium, being diagnosed at an early stage and thereby having a better prognosis than typical endometrioid carcinoma. To avoid confusion with papillary serous carcinoma, some authors refer to this entity as endometrioid adenocarcinoma with papillary architecture(16). In mixed endometrial adenocarcinomas the tumor has microscopic features intermediate between serous and endometrioid; for example, papillary architecture typical of serous but without classical cytologic features or glandular architecture with epithelial features suggestive of serous (11). Villoglandular Cervical Adenocarcinoma (VGCA) is a rare well-differentiated subtype of endocervical mucinous adenocarcinoma that usually occurs in young women in early clinical stage I-II [18-24]. VGCA is associated with a better prognosis than other adenocarcinomas [18-20,23,25-27] аnd accounts for 5% of cervical adenocarcinomas [28,29]. It is characterized as superficially infiltrative and shows infrequent lymph node invasion and lymphovascular space invasion [30,31]. Serous Carcinoma of the Uterine Cervix (SCUC) is a very rare malignant tumor, while this histological subtype is common in the ovary, fallopian tube, uterine corpus and peritoneum. Because of its rarity, details of the clinicopathological features of SCUC are largely unknown [32]. SCUC has a complex papillary pattern with epithelial stratification. The tumor is characterized by >10 mitotic figures per 10 high-power fields. An intense acute and chronic inflammatory infiltrate is typically present within the cores of the papillae and in areas of stromal invasion [33]. Pleomorphic and macronucleated atypical cells are observed [32]. Occasional psammoma bodies are present, like in the presented clinical case (Figure 1). The tumor cells in SCUC are positive for p16, focally positive for p53 and ER, and negative for progesteron receptor. Age <65 years, stage >I, tumor size >2 cm, tumor invasion >10 mm, the presence of lymph node metastases, and elevation of serum CA-125 were associated with a poor prognosis [33].
Important for the DD between endometrial and endocervical carcinoma is the study which notes that p16 reactivity is mainly limited to areas with endometrioid or nonspecific cell type differentiation and does not occur in areas with normal endocervical mucinous morphology [34]. It is important to recognize special variants such as uterine serous carcinoma and clear cell carcinoma, as these can show extensive staining for p16 [34]. Univariate analysis on the training set identified 6 markers—p16, ProExC, ER, PR, vimentin, and HPV ISH—as performing well in distinguishing between endocervical and endometrial origin. Positive results for vimentin, ER, and PR support endometrial origin whereas p16, ProExC, and HPV ISH support endocervical origin [34,35]. Primary endocervical adenocarcinomas are characterized by CEA positivity, which is usually, but not always diffuse, negativity for vimentin, and negativity or focal weak positivity for ER [35]. Similar to serous carcinomas, all endocervical adenocarcinomas exhibited diffuse/moderate-strong p16 expression, with percentage of positive tumor cells ranging from 90% to 100% [12]. In the distinction between a high-risk HPV-related (usual type) endocervical adenocarcinoma and a low-grade endometrial endometrioid adenocarcinoma, the most useful immunohistochemical markers are p16 and ER and PR hormone receptors [36]. Given the limits of immunohistochemical stains in distinguishing between endocervical and endometrial origin, individual cases should be evaluated in the context of standard clinical, radiographic, gross, and morphologic findings [34]. In the presented clinical case, DD is required between synchronous serous adenocarcinomas, i.e. primary endometrial with infiltration in the uterine cervix and primary tubo-ovarian carcinoma, or serous endometrial carcinoma with metastases in both ovaries. Multifocal tumor distribution is relatively common in carcinomas arising in the female genital tract. Accurate designation of tumor origin is not always straightforward but is important for staging, prognosis and management [36]. In the context of endometrial carcinoma, the most common dilemmas occur in the setting of concurrent endometrial and adnexal/peritoneal high-grade serous adenocarcinoma [3]. High grade serous tubo-ovarian adenocarcinomas are characterized by complex papillary, solid or glandular architecture [6]; Significant nuclear atypia; significant nuclear pleomorphism (>3 x variation in size) with large, bizarre and multinucleated forms [37]. Prominent nucleoli, often large and eosinophilic; High mitotic index: ≥ 12 mitotic figures per 10 high power fields, often atypical; Necrosis is frequent [13]. Positive stains: p16 (~60%), ER (80%), PR (30%), high Ki67 proliferation index (> 75%) [38]. Usually they present as unilateral tubo-ovarian tumor in 80-90% of cases, no tumor infiltration on the surface of the ovary; without invasion into the lumen of the tube; without vascular invasion - 71.4%; without or presence of ovarian endometriosis [39,40]. IHC analysis of ER and PR of endometrioid endometrial and ovarian tumors distinguishes primary tumor synchronicity from hematogenous metastasis. The same levels of ER and PR are found in metastatic endometrial or ovarian cancer (Figure 5C,5D) and different in synchronous primary endometrial and ovarian neoplasms [41,42]. One general principle used in determining tumor origin in pathology is the identification of intraepithelial carcinoma because the presence of such a lesion is considered a strong evidence of tumor development at a particular anatomic site. Occasionally metastases can colonize preexisting mucosal structures and replace the native epithelium, for example, endometrial serous adenocarcinoma may involve the mucosa of the fallopian tube and closely mimic serous tubal intraepithelial carcinoma [3]. Simultaneous endometrioid carcinomas of the uterus and of the fallopian tube are unusual and occur primarily in obese perimenopausal women. These tumors are predominantly well or moderately differentiated with dissimilar endometrial and fallopian tube grades. The carcinoma of the fallopian tube is usually unilateral and located at the distal end of the tube [36]. It is well established that high-grade endometrial carcinomas may present with metastases even when there is minimal myometrial invasion [3].
Conclusions from the IHC panel of the presented clinical case: 1/The positive expression of ER and PR receptors in the tumor cells of the tumor in the cervix proves the primary endometrial origin of the carcinoma with infiltration of the cervical canal (Figure 5A,5B,5D,5E). 2/ Diffuse positive expression of p16 in the tumor cells of the endometrial tumor mass and in the cervical tumor is typical for serous carcinomas (Figure 4). 3 / Positive expression for Vimentin in the tumor cells in the cervical and the ovarian tumors is typical for primary endometrial carcinoma, negative - is observed in primary viloglandular endocervical adenocarcinoma and primary endometrioid ovarian carcinomas. The positive expression of Vimentin in both tumors of the cervix and the ovary proves the local growth of serous endometrial carcinoma to the cervix with bilateral metastases in the ovaries (Figure 6). 4/ IHC analysis of ER and PR of the endometrial and the ovarian tumors distinguishes primary tumor synchronicity from hematogenous metastasis. The same levels of ER and PR in the endometrial and ovarian tumors report bilateral metastatic tumor spread in the ovaries (Figure 5). 5/ Positive EMA expression in cervical tumor cells determines a poor prognosis (Figure 7). 6/ Psammoma bodies are observed on histological examination of the tumors of the cervix, uterine body, fallopian tube (Figure 1,3A,3B,5A,5B). They are typical for malignancies such as serous carcinoma of the ovaries and serous carcinomas of the cervix, endometrium, fallopian tube, peritoneum [43].
By the above immunohistochemical characteristics primary locally advanced serous endometrial carcinoma/ III B class stage is evidenced. Despite the initial tumor infiltration in the myometrium, as well as the superficial invasion of the cervix in the presented clinical case, the typical tumor spread of locally advanced serous carcinoma with unilateral infiltration into the right fallopian tube and left parametrium and bilateral ovarian metastases are observed.
Prognosis
Uterine Papillary Serous Carcinoma (UPSC) is a rare aggressive variant of type II endometrial cancer [44]. The prognosis is unfavorable, as it is an extremely aggressive tumor with a high potential for local recurrences and distant metastases. The recurrence rate of UPSC is high, estimated to be 31%–80% even in its early stages (stage I–II) [45]. Frequent recurrences in UPSC have led to the induction of adjuvant treatment, including systemic chemotherapy or radiation therapy [46]. Prognosis of the UPSC is affected by age, stage, and histology as well as treatment [47]. UPSC accounts for 10% of all endometrial cancer; however, it carries the poorest prognosis, with 5-year survival rates as low as 55% [48,49].
Complex Treatment
With such a dismal prognosis, these patients should be treated aggressively [48]. The primary treatment is surgical. FIGO-cancer staging is done at the time of surgery which consists of peritoneal cytology, total hysterectomy, bilateral salpingo-oophorectomy, pelvic/para-aortic lymphadenectomy, and omentectomy. After staging and surgery, radiation therapy and/or chemotherapy is recommended to treat patients at high risk for recurrence [50]. Analyses of Gynecologic Oncology Group (GOG) protocol 209 (a noninferiority trial in advanced/recurrent endometrial cancer patients comparing carboplatin and paclitaxel vs paclitaxel, adriamycin and cisplatin) support the favorable side effect profile of six cycles of carboplatin (AUC 6) and paclitaxel (175 mg/m2) [51]. Those who are identified to have residual UPSC in the uterus at the time of surgery, should receive adjuvant carboplatin and paclitaxel chemotherapy, and a strong consideration should be given for vaginal cuff brachytherapy [48]. UPSC’s aggressive features, resistance to chemotherapy, poor prognosis, and extremely high recurrence rate (50% to 80%) contribute to the increase in endometrial cancer-related deaths every year. Currently, surgery together with chemotherapy and radiotherapy (vaginal cuff brachytherapy or external pelvis radiotherapy) remains the dominant treatment option for UPSC [1].
Conclusion
We present a rare clinical case of locally advanced uterine papillary serous carcinoma with difficult differential diagnosis both in terms of histogenesis and histological features, as well as in terms of its primary origin, which is extremely important for complex treatment. Strict pathohistological and immunohistochemical analysis is required for the differential diagnosis of this rare aggressive variant of type II endometrial carcinoma. The prognosis is unfavorable, as it is an extremely aggressive tumor with a high potential for local recurrences and distant metastases. The primary treatment is surgical. FIGO-cancer staging is done at the time of surgery which consists of peritoneal cytology, total hysterectomy, bilateral salpingo-oophorectomy, pelvic/para-aortic lymphadenectomy and omentectomy. Locally advanced uterine serous carcinomas require adjuvant treatment, including concominant radiochemotherapy followed by systemic chemotherapy.
References:
- Li Zhang, Suet Ying Kwan, Kwong Kwok Wong, et al. Pathogenesis and Clinical Management of Uterine Serous Carcinoma. Cancers (Basel). 2020;12(3):686.
- Creasman WT, Eddy GL. Recent advances in endometrial cancer. Semin. Surg. Oncol. 1990;6:339-342.
- Colin JR Stewart, Christopher P Crum, W Glenn McCluggage, et al. Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites. Int J Gynecol Pathol. 2019;38(1):S75-S92.
- Dominik Denschlag, Uwe Ulrich, Günter Emons. The Diagnosis and Treatment of Endometrial Cancer. Progress and Controversies. Dtsch Arztebl Int. 2011;108(34-35):571-577.
- Parra-Herran C. Staging-carcinoma and carcinosarcoma. 2020. PathologyOutlines.com website. http://www.pathologyoutlines.com/topic/uterusstaging.html.
- Schulte JJ, Lastra RR. Serous carcinoma. 2020. PathologyOutlines.com website. http://www.pathologyoutlines.com/topic/uterusserous.html.
- Hendrickson M, Ross J, Eifel P, et al: Uterine papillary serous carcinoma: A highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol 1982;6:93-108.
- Chen J, Trost DC, Wilkinson EG. Endometrial papillary adenocarcinomas: Two clinicopathologic types. Int J Gynecol Pathol 1985;4:279.
- Zheng W, Chambers SK. Histopathology of endometrial hyperplasia and endometrial carcinoma. An update by Dr LC Horn et al in Annals of Diagnostic Pathology 2007;11:297-311. Ann. Diagn. Pathol. 2008;12:231-232.
- Nakayama K, Nakayama N, Ishikawa M, Miyazaki K. Endometrial serous carcinoma: Its molecular characteristics and histology-specific treatment strategies. Cancers. 2012;4:799-807.
- Desouki M. Mixed carcinomas. 2020. Pathology Outlines.com website. http://www.pathologyoutlines.com/topic/uterusmixedcarcinoma.html.
- Anna Yemelyanova , Hongxiu Ji, Ie-Ming Shih, et al. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol 2009;33(10):1504-1514.
- Forgó E, Longacre TA. High grade serous carcinoma. 2020. PathologyOutlines.com website. http://www.pathologyoutlines.com/topic/ovarytumorserouscarcinomahg.html.
- Pernick N. Epithelial Membrane Antigen (EMA). 2020. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsema.html.
- Philip B Clement , Robert H Young. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol 2004;11(3):117-142.
- Gordon M, Ireland K. Pathology of Endometrial Carcinoma. Glob. libr. women's med., (ISSN: 1756-2228) 2008; DOI 10.3843/GLOWM.10238.
- Zaino RJ, Kurman RJ, Brunetto VL, et al. Villoglandular adenocarcinoma of the uterus: a clinicopathologic study of 61 cases. A Gynecologic Oncology Group Study. Am J Surg Pathol 1998;22:1379-1385.
- Dede M, Deveci G, Deveci MS, et al. Villoglandular papillary adenocarcinoma of the uterine cervix in a pregnant woman: a case report and review of literature. Tohoku J Exp Med. 2004;202(4):305-310.
- Takai N, Hayashita C, Nakamura S, et al. Villoglandular papillary adenocarcinoma of the uterine cervix diagnosed during pregnancy. Eur J Gynaecol Oncol. 2010;31(5):573-574.
- Falcón O, García R, Lubrano A, et al. Successful term delivery following conservative management for villoglandular papillary adenocarcinoma of the uterine cervix: A case report. Gynecol Oncol. 2006;101(1):168-171. Epub 2005 Dec 2.
- Lu FH, Chen BF, Yang YC. Well-differentiated papillary villoglandular adenocarcinoma of the uterine cervix: a case report. Zhonghua Yi Xue Za Zhi (Taipei); 1998;61(7):436-440.
- Young RH, Scully RE. Villoglandular papillary adenocarcinoma of the uterine cervix. A clinicopathologic analysis of 13 cases. Cancer. 1989; 63: 1773-1779.
- Jones MW, Silverberg SG, Kurman RJ. Well-differentiated villoglandular adenocarcinoma of the uterine cervix: a clinicopathological study of 24 cases. Int J Gynecol Pathol. 1993;12:1-7.
- Yamazawa K, Matsui H, Seki K. et al. Human papillomavirus-positive well-differentiated villoglandular adenocarcinoma of the uterine cervix: A case report and review of the literature. Gynecol Oncol. 2000;77:473-477.
- Jerry Cheng-Yen Lai, Jen-Ruei Chen, Yu-Jen Chen, еt al. Villoglandular Adenocarcinoma of the Uterine Cervix: An Analysis of 12 Clinical Cases. 2011. http://dx.doi.org/10.1016/j.ijge.2011.01.009.
- Stanley-Christian H, Heim BK, Hines JF, et al. Villoglandular adenocarcinoma of the cervix: a report of three cases and review of literature. Gynecol Oncol 1997;66(2):327-330.
- Korach J, Machtinger R, Perri T, et al. Villoglandular papillary adenocarcinoma of the uterine cervix: a diagnostic challenge. Acta Obstet Gynecol Scand. 2009;88:355-358.
- Wolter Kluster. Villoglandular Adenocarcinoma of the Endometrium and its Differential Diagnosis With Uterine Serous Carcinoma. Pathology Case Revies 2000;5(3):163-167.
- Wells M, Ostor AG, Crum CP, et al. Tumors of the cervix. in: F.A. Tavassoli, P. Devilee (Eds.) Tumors of the Breast and Female Genital Organs. IARC Press, Lyon, France; 2003: 262-276.
- Macdonald RD, Kirwan J, Hayat K, et al. Villoglandular adenocarcinoma of the cervix: clarity is needed on the histological definition for this difficult diagnosis. Gynecol Oncol. 2006;100(1):192–194.
- Costa MJ, Mclinay KR, Trelford J. Cervical carcinoma with glandular differentiation: histological evolution predicts disease recurrence in clinical stage I or II patients. Hum. Pathol. 1995;26(8):829-837.
- Shoko Kitade, Kazuya Ariyoshi, Kenichi Taguchi, et al. Serous carcinoma of the uterine cervix: Clinicopathological features differing from serous carcinomas of other female organs. J. Obstet. Gynaecol. Res. 2020;46(1):153–160.
- C Zhou, C B Gilks, M Hayes, P B Clement. Papillary serous carcinoma of the uterine cervix: a clinicopathologic study of 17 cases. Am J Surg Pathol, 1998;22(1):113-120.
- Christina S Kong, Andrew H Beck, Teri A. Longacre. A Panel of 3 Markers Including p16, ProExC, or HPV ISH is Optimal for Distinguishing Between Primary Endometrial and Endocervical Adenocarcinomas. Am J Surg Pathol. 2010;34(7):915-926.
- McCluggage WG, Sumanthi VP, McBride HA, et al. A Panel of Immunohistochemical Stains, Including Carcinoembryonic Antigen, Vimentin, and Estrogen Receptor, Aids the Distinction between Primary Endometrial and Endocervical Adenocarcinomas. Int JGyn Pathol 2002;21(1):11-15.
- Culton LK, Deavers MT, Silva EG, et al. Endometrioid carcinoma simultaneously involving the uterus and the fallopian tube: a clinicopathologic study of 13 cases. Am J Surg Pathol 2006;30:844-849.
- Chih-Yi Hsu , Robert J Kurman, Russell Vang, et al. Nuclear size distinguishes low- from high-grade ovarian serous carcinoma and predicts outcome. Hum Pathol 2005;36(10):1049-1054.
- Ming Chen , Shuzhong Yao, Qinghua Cao, et al. The prognostic value of Ki67 in ovarian high-grade serous carcinoma: an 11-year cohort study of Chinese patients. Oncotarget 2016;8(64):107877-107885.
- Karki S, Chapagain U. Synchronous primary tumors of the endometrium and ovary. J Path Nepal 2012;2, 189-192.
- Scully RE, Young RH, Clement PB, et al. Atlas Pathology, Tumors of the ovary, maldeveloped gonads, Fallopian tube, and broad ligament. 3-rd Series, Fascicle 23. Armed Forces Institute of Pathology. Washington,DC;1998:126.
- Dordevic M, Mitrovic S, Jovanovic B. Sinhroni tumori uterusa- prikaz slucaja :ed Pregl 2010;LXIII (7-8):570-573.
- Halperin R, Zehavi S, Hadas E, et al. Simultaneous carcinoma of the endometrium and ovary vs endometrial carcinoma with ovarian metastases: a clinical and immunohistochemical determination. Int J Gynecol Cancer. 2003;13(1):32-37.
- Hasteh F. Psammoma bodies. 2020. Pathology Outlines.com website. https://www.pathologyoutlines.com/topic/cervixcytologypsammomabodies.html.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: a review of the literature. Gynecol Oncol. 2012;127(3):651-661.
- Keisei Tate, Hiroshi Yoshida, Mitsuya Ishikawa, et al. Prognostic factors for patients with early-stage uterine serous carcinoma without adjuvant therapy. J Gynecol Oncol. 2018;29(3):e34.
- Goldberg H, Miller RC, Abdah-Bortnyak R, et al. Outcome after combined modality treatment for uterine papillary serous carcinoma: a study by the Rare Cancer Network (RCN). Gynecol. Oncol. 2008;108(2):298–305.
- Jonathan D Black, Diana P English, Dana M Roque, and Alessandro D Santin. Targeted therapy in uterine serous carcinoma: an aggressive variant of endometrial cancer. Womens Health (Lond Engl). 2014;10(1):45-57.
- Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer. 2006;94(5):642-646.
- ClinicalTrials.gov Identifier: NCT04030000. Uterine Serous Carcinoma and Adjuvant Combined Intraperitoneal Chemotherapy and Radiation.
- Miller D, Filiaci V, Fleming G, et al. Randomized Phase III noninferiority trial of first-line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study (late-breaking abstract); Presented at: Annual Meeting of the Society of Gynecologic Oncologists. Austin, TX, USA; 2012;24-27.

