Case Report of Disseminated Pseudomonas Infection with Superadded Burkholderia Infection – A Battle Lost!
Sangita K*, Manish K, Murari B and Ashok S
Department of General Medicine, Tata Main Hospital, Tata Steel, Jamshedpur, India
Received Date: 14/09/2020; Published Date: 03/11/2020
*Corresponding author: Sangita Kamath, Senior Consultant, Department of General Medicine, Tata Main Hospital, Duplex-25, Vijaya’s Heritage, Anil Sur Path, Uliyan, Kadma, Jamshedpur – 831005, Jharkhand, India. Tel: 07763807130; E-mail: dr.sdkamath@tatasteel.com; dr.sdkamath@gmail.com
Abstract
Pseudomonas aeruginosa is a gram-negative pathogen, that often causes nosocomial pneumonia in hospitalized patients. Most of these patients have risk factors for pseudomonas infection. Although uncommon, therehave been case reports of previously healthy individuals who developed Community-Acquired Pneumonia (CAP) caused by P. aeruginosa. Such cases have often rapidly progressive course and prove fatal. We, hereby, report a case of pseudomonas pneumonia in a 29-year-old immunocompetent patient, who developed disseminated infection and superinfection with yet another nosocomial pathogen, Burkholderiacepacia, eventually leading to septic shock and death, despite appropriate antibiotic therapy.
Keywords: Infection; Bacteremia; Pneumonia; Antibiotics; Healthy
Introduction
Community-Acquired Pneumonia (CAP) due to pseudomonas aeruginosa in a previously healthy individual is rare. Pseudomonas is a gram-negative, aerobic organism which generally infects patients with structural lung diseases like cystic fibrosis and Chronic Obstructive lung Disease (COPD) and immunocompromised host [1]. We report a rare case of P. aeruginosa CAP in a young female without risk factors that progressed to necrotizing pneumonia, with formation of cavities. Further, there was dissemination of infection to other organs resulting in metastatic abscesses in liver and spleen, superinfection with Burkholderiacepacia, eventually leading to septic shock, Multi-Organ Dysfunction Syndrome (MODS) and death, despite being treated with appropriate antibiotics.Such a case of disseminated pseudomonas infection is hitherto not described in the literature.
Case Report
A 22-year-old previously healthy lady was admitted to our hospital with history ofhigh-grade fever for 7 days accompanied by cough, productive of scanty yellowish sputum for 5 days. She had also noticed swelling of both legs, three days prior to the admission. She had undergone caesarian section 3 months prior and remained asymptomatic till 1 week prior to hospital admission. She did not have past history of any significant medical ailment and addictions. On examination, she had pallor and bilateral pitting pedal edema. There was no cyanosis, clubbing or generalized lymphadenopathy. She was febrile, pulse rate was 124/minute, regular, blood pressure was 112/70 mm Hg and respiratory rate was 22/minute with accessories working. Breath sounds were vesicular type and were diminished at both bases. SaO2 while breathing ambient air was 92%. Abdomen was fatty and there was no organomegaly. Other systems were normal.
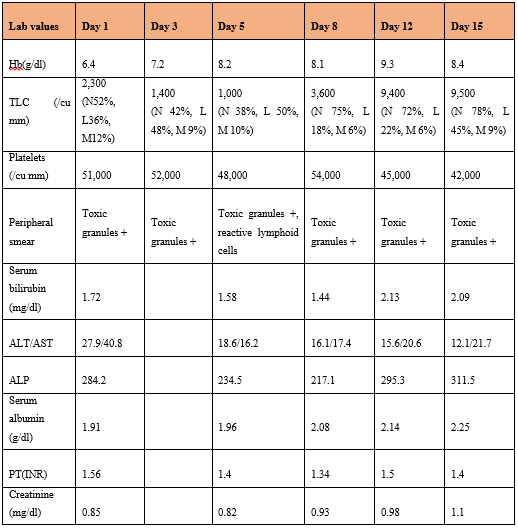
On admission, her hemoglobin was 6.4gm/dl, mean corpuscular volume (MCV) 77.8 fl, WBC count 2,300/µL (52% neutrophils with toxic granules, 36% lymphocytes, 12% monocytes) and platelet count 51,000/cu mm. Evaluation of the cause of anemia revealed serum iron – 34.8mcg/dl, serum ferritin – 564 mcg/L, serum folic acid –5.6ng/ml, serum B12 - 442pg/ml, reticulocyte count of 0.8%, normal adult pattern hemoglobin electrophoresis and negative Direct Coomb’s Test (DCT). Renal function test was normal. Liver function test revealed serum bilirubin – 1.72 mg/dl, alanine transferase (ALT)-27.9U/L, Aspartate Transferase (AST) 40.8U/L, alkaline phosphatase (ALP)-284.4U/L, total serum protein 6.1g/dl, serum albumin- 2.07 g/dl, serum globulin – 4.03g/dl. Her coagulation tests revealed prothrombin time–13.2, control-11 secs, International Normalized Ratio (INR) 1.54 and activated partial thromboplastin time – 26.9 sec, control-24.6. C- Reactive Protein (CRP)was 29.24 mg/dl. Her viral markers (HBsag, HIV 1 and ll and HCV antibodies) were non-reactive. Her antinuclear antibodies (ANA) and antibodies to double-stranded DNA (dsDNA) were negative. Mantoux skin test was negative. Urine routine examination showed 10 to 12 pus cells/ high power field and 274.6mg/dl of protein.Her serial laboratory investigations were as tabled in the Table 1.
Table 1: Serial laboratory parameters from the day of admission.
Chest radiograph showed left mid zone pneumonia (Figure 1) while Contrast Enhanced Computed Tomography (CECT) of thorax showed multiple small nodules and cavities in both lungs with mild left pleural effusion (Figures 2a-2d). Echocardiography was normal. Abdominal ultrasound showed hepatosplenomegaly with imaging features suggestive of multiple abscesses in liver largest measuring 6 cm x 4 cm and largest splenic abscess measuring 4 x 3.5 cm with moderate ascites while Contrast Enhanced Computed Tomography (CECT) abdomen confirmed the above findings and additionally showed few small abscesses in the right kidney (figures 3a-3d).
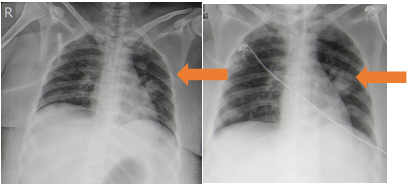
Figure 1a & b: Chest radiograph showing left mid zone pneumonia (on admission and 4 days later).
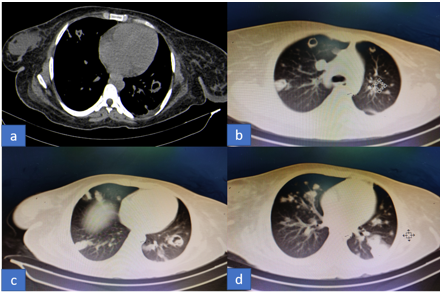
Figures 2a-d: CECT thorax (bone and lung windows) showing bilateral multiple nodular and cavitary lesions.
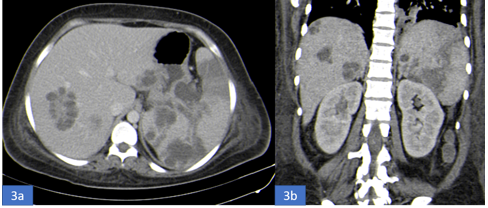
Figure 3a-b: a. CECT abdomen (axial view) showing multiple abscesses in the liver and spleen.
b.CECT abdomen (coronal section) showing multiple abscesses in the liver, spleen and kidney.
Her blood culture sent on the day of admission grew gram negative bacilli, pseudomonas aeruginosa (BACTEC method), which showed sensitivity to meropenem, imipenem, ciprofloxacin and piperacillin-tazobactum and resistance to amikacin, colistin, cefepime, cefaperazone-sulbactum and tigecycline. She was treated with intravenous piperacillin-tazobactum 4.5 g thrice a dayand levofloxacin 500 mg once a dayfor 10 days with other supportive measures. However, she continued to get high grade fever spikes (104 to 105F). A second blood culture sent on day 8 of admission grew Burkholderiacepacia sensitive to meropenem and ciprofloxacin. Her antiobiotic therapy was escalated to intravenous meropenem 1 g tidand oral trimethoprim/ sulfamethoxazole (80/400 mg). She, however, went into septic shock needing double vasopressor support. Shelater developed multiorgan dysfunction syndrome (MODS) and Disseminated Intravascular Coagulation (DIC) and succumbed to her illness. A final diagnosis of community acquired pseudomonas pneumonia with dissemination with metastatic abscesses with superadded Burkholderia infection leading to MODS was made.
Discussion
This patient was diagnosed to have severe Community-Acquired Pneumonia (CAP) due to P. aeruginosa, which was complicated by secondary hematogenous spread causing metastatic abscess in liver and spleen and MODS involving cardiovascular, hematologic and respiratory systems. Injury to thealveolar epithelium causes release of proinflammatory cytokines into the circulation which are ultimately responsiblefor septic shock. During treatment, she developed superadded secondary infection with Burkholderiacepacia. Pseudomonas aeruginosa is a gram-negative aerobic bacterium that causes several types of infections including wound, urinary tract, and respiratory tract infections. It is a common pathogenicbacteria in hospital acquired pneumonia, and is usually seen in patients who have structural lung disease, or are immunocompromised [1]. However, it is rarely identified as a cause of pneumonia in previously healthy individuals, accounting for 0.4–6.9% of the reported cases of CAP requiring hospitalization [2] and 1.8–8.3% CAP requiring Intensive Care Unit (ICU) admission [3]. Several studies [4,5] found that fatal P. aeruginosa pneumonia in healthy patients was associated with exposure to contaminated hot tubs. Harris et al.[6] reported a case of P. aeruginosapneumonia in a chronic asthmatic patient who was using a home humidifying device that contained water contaminated with P. aeruginosa.Other risk factors identified in various studies include bronchiectasis, cystic fibrosis, chronic heart failure, smoking, malnutrition and immunocompromised status [7]. Our patient was a home maker and there was no history of any of the above risk factors. Few studies have reported prior infection with Influenza A to be a risk factor for P. aeruginosa infection. Influenza viral infection causes respiratory epithelial cell dysfunction and apoptosis by disruption of protein synthesis and thus allowing increased bacterial adherence and invasion [1,8]. Occupational exposure in health care workers may also be a risk factor [9]. Our patient, prior to hospital admission, did not have any risk factor for pseudomonas infection. Compared to pneumonia caused by other pathogens,P. aeruginosa CAP runs a fulminant course, shows rapid dissemination and has poor prognosis[1].Semerci SYet al reported a case of hepatic and splenic abscess in a preterm infant caused by P.aeruginosa[11].Govan et al reported a case of a previously healthy 49-year-old man who died within 12 hoursofhospital admission. P. aeruginosa was subsequently isolated both from sputum and blood samples taken at the time of admission and from postmortem specimens of his lung, liver and spleen [2,12].
In a review of literature by Hatchette et al from 1966 to 2000, he reported 11 cases of P aeruoginosa[10]. Further Maharaj S et al did a review of nine cases indexed in PubMed from 2001to 2016 of which 3 were complicated by sepsis with MODS [2]. However, none of these cases were complicated by metastatic abscess formation.Predilection for the Right Upper Lobe (RUL) was seen in two-thirds of the cases of P. aeruginosaCAP described by Hatchette et al[10] and in 7 out of 9 cases by Maharaj S et al [2]. P. aeruginosais an aerobic bacterium and upper lobes provide a more favorable environment due to higher ventilation to perfusion ratios [2]. However, our patient had bilateral, multifocal involvement with cavitation in CT thorax while the chest radiograph initially showed only left mid zone involvement. As P.aeruoginosa is an invasive pathogen, and causes thrombotic endarteritis, pneumonia rapidly progresses to necrosis, with coalescing of these foci into cavities. Surprisingly the total leucocyte count was normal on admission, despite the fact that the infection was disseminated. However, there was neutrophilic leukocytosis with toxic granules seen in the neutrophils and CRP was very high suggestive of bacterial infection.Later, she went to develop neutropenic sepsis. The leucocyte count may be variable (high, normal or low) as reported by Hatchette et al [10].The current guidelines from the Infectious Diseases Societyof America(IDSA) suggest that patients with CAP who are hospitalizedin the ICU and or have risk factors like structural lung diseases, immunocompromised status, prior use of antibiotics before admission, should receive a macrolide or a fluoroquinoloneplus cefotaxime or ceftriaxone or a beta-lactam–beta-lactamase inhibitor [13].Our patient was treated as per the protocol for treatment of pseudomonas pneumonia with antipseudomonal antibiotic(piperacillin-tazobactum and clarithromycin which was escalated to meropenem, clarithromycin and co-trimoxazole), considering the possibility of emergence of resistant strains during the therapy. This was also in accordance with the antibiotic sensitivity pattern. Though earlier studies showed better prognosis in younger patients, ourpatient unfortunately did not respond to the treatment.
cepacia is a member of a group known as B. cepacia complex which includes nine differentrecognized genomovars. It is a gram-negative, glucose-non-fermenting, aerobic bacillus and causes acute systemic infection and “cepacia syndrome” which is progressive necrotizing pneumonia [14].It is associated with infections in patients with underlying lung disease, like cystic fibrosis, chronic granulomatous disease, immunocompromised individuals, hospitalized patients and drug addicts [15].Outbreaks may occur due to contaminated antiseptics, disinfectants, ventilators and other types of medical equipment [14]. Also, person-to-person spread has also been documented.In a study by Bressler et al, among ICU patients without cystic fibrosis, bacteremia due to B. cepacia was associated with renal failure requiring dialysis, recent abdominal surgery, bronchoscopies, tracheostomy, and presence of a central line [16]. In a study by Huang et al, most patients had seriouscomorbidities including diabetes mellitus, Chronic Obstructive Pulmonary Disease (COPD), malignancy and congestive heart failure [15]. Our patient had central line insertion and was transferred to Intensive Care Unit (ICU). She,thus having two risk factors.The organism was sensitive to meropenem and co-trimoxazole. As she had overwhelming infection, resulting in metastatic infection in liver, splenic and renal infection, she succumbed to her illness.To our knowledge, this is the first report of pseudomonas pneumonia complicated by metastatic abscesses (liver, spleen and kidney), secondary infection with B. cepacia and MODS.
Conclusion
Pseudomonas aeruginosa, can cause CAP. The disease, although described in immunocompromised patients and with underlying risk factors, can also occur in apparently healthy individuals. The initial symptoms may not be precise enough for a specific diagnosis. Though earlier studies have shown predilection for right upper zone, pseudomonas pneumonia can be bilateral and tend to become necrotizing with cavity formation. CT thorax can demonstrate coalescing of infectiousfoci into cavities and is recommended when the initial radiograph is not contributory. The infection can disseminate to other organs as in our case and ultimately lead to septic shock despite appropriate antibiotic therapy. Superadded infection with other pathogens can also occur. Physicians must, therefore, keep this possibility in mind while diagnosing CAP, especially if there is associated septic shock and treat the patient early with anti-pseudomonal antibiotics.
Consent
Written consent was taken from the patient during the hospital stay for the publication of the case and pictures.
References:
- Wang T, Hou Y and Wang R. A case report of community-acquired Pseudomonas aeruginosa pneumonia complicated with MODS in a previously healthy patient and related literature review.BMC Infectious Diseases. 2019;19:130-6.
- Maharaj S, Isache C, Seegobin K,Chang S, and Nelson G. Necrotizing Pseudomonas aeruginosaCommunity-AcquiredPneumonia: A Case Report and Review of the Literature. Case Reports in Infectious Diseases. 2017;1717492:1-5.
- Cillóniz G, Gabarrús A, Ferrer M, de la Bellacasa JP, Rinaudo M, Mensa J, et al. Community-acquired pneumonia due to multidrugandnon-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-25.
- Huhulescu S, Simon M, Lubnow M, Kaase M, Wewalka G, Pietzka AT,et al. Fatal Pseudomonas aeruginosa pneumonia in a previously healthy woman was most likely associated with a contaminated hot tub. Infection. 2011;39(3):265-9.
- Crnich CJ, Gordon B and Andes D. Hot tub-associated necrotizing pneumonia due to pseudomonas aeruginosa. Clin Infect Dis. 2003;36(3):e55-7.
- Harris AA, Goodman L and Levin S. Community acquired Pseudomonas aeruginosapneumonia associated with the use of a home humidifier. West JMed. 1984;141:521-3.
- Sibila O, Laserna E, Maselli DJ, Fernandez JF, Mortensen EM,Anzueto A, et al. Risk factors and antibiotic therapy in P.aeruginosa community-acquired pneumonia. Respirology. 2015;20(4):660-6.
- Chertow DS andMemoli MJ. Bacterial coinfection in influenza: A grand roundsreview. JAMA. 2013;309(3):275-82.
- Cirigliano MD andGrippi MA. Overwhelming pneumonia in a healthy young nursing assistant. Hosp Pract (Off Ed). 1994;29:31-4.
- Hatchette TF, Gupta R, and Marrie TJ. Pseudomonas aeruginosa community-acquired pneumonia in previously healthy adults: Case report and review of the literature. ClinInfect Dis. 2000;31(6):1349-56.
- Semerci SY, Babayigit A, Cebeci B, Buyukkale G and Cetinkaya M. Hepatic Abscesses in Preterm Infants: Reportof Three Cases and Review of the Literature. Journal of Tropical Pediatrics. 2016;62:255-60.
- Govan J, Reiss-Levy E and Bader L. Pseudomonas pneumonia with bacteremia. Med J Aust. 1977;1:627-8.
- Bartlett JG, Breiman RF and Mandell LA. Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811-38.
- Matthaiou DK, Chasou E, Atmatzidis S andTsolkas P. A case of bacteremia due to Burkholderiacepacia in a patient without cysticfibrosis. Respiratory Medicine CME. 2011;4:144-5.
- Huang CH, Jang TN, Liu CY, Fung CP, Yu KW and Wong WW. Characteristics of patients with Burkholderiacepacia bacteremia. J Microbiol Immunol Infect. 2001;34:215e9.
- Bressler AM, Kaye KS, LiPuma JJ, Alexander BD, Moore CM, Reller LB, et al. Riskfactors for Burkholderiacepacia complex bacteremia among intensive care unitpatients without cystic fibrosis: a case-control study. Infect Control Hosp Epidemiol. 2007;28:951e8.

