Feasibility of a Combined TAVI and Aorfix EVAR under Local Anesthesia in a Patient with Severe Aortic Valve Stenosis and Tortuous 8.4cm AAA
Can Hazar*11, Olanrewaju Ogunleye*2, Rosemary Darwood3, Manik Chandra4, Christopher Malkin5 and Sapna Puppala*1
1Specialist Registrar in Interventional Radiology, United Kingdom
2Department of Vascular Radiology, ESOR IR visiting scholar, United Kingdom
3Department of Vascular Surgery, Consultant Vascular Surgeon, United Kingdom
4Department of Anesthetics, Consultant Vascular Anesthetist, United Kingdom
5Department of Cardiology, Consultant Interventional Cardiologist, United Kingdom
6Consultant Vascular Intervention list, Leeds Teaching Hospital NHS trust, United Kingdom
Received Date: 14/09/2020; Published Date: 13/10/2020
*Corresponding author: Sapna Puppala, B Floor, Jubilee Wing, Leeds General Infirmary, Great George Street, Leeds LS1 3EX, United Kingdom. Can Hazar, Specialist Registrar in Interventional Radiology, United Kingdom. Tel: 447787044534; E-mail: canhazar@doctors.org.uk
Abstract
Endovascular Aneurysm Repair (EVAR) is a routinely used minimally-invasive treatment technique for Abdominal Aortic Aneurysm (AAA). Trans catheter Aortic Valve Implantation (TAVI) is widely accepted as an alternative to surgical valve replacement in elderly or high risk patients with aortic stenosis. Aortic aneurysm and aortic stenosis may coexist in an older age group often with other comorbidities. Combined procedures increase complexity and are thought to increase morbidity and mortality compared to sequential procedures. However in some circumstances the risk of a combined TAVI and EVAR may reduce cumulative risk compared to sequential procedures [1]. We describe the case of a 79 years old male patient with incidental findings of asymptomatic AAA and severe Aortic Stenosis (AS) who underwent successful simultaneous TAVI and EVAR under local anesthesia.
Keywords: TAVI; EVAR; Percutaneous; Local Anesthesia
Case Presentation
A 79-year-old male was referred to our tertiary unit due to an incidental finding of a pulsatile abdominal mass. He was an ex-smoker with a previous history of transient ischemic attack and left carotid endarterectomy. Co-morbidities included Type II diabetes mellitus and previous asbestos exposure. Computed Tomogram (CT) angiography showed an 8.4 cm infra-renal aortic aneurysm with a narrow (18mm) and angulated (73 degrees) neck (Figure 1). CT also showed extensive arteriosclerosis involving the coronary arteries, abdominal aorta, both iliac and femoral arteries.
Echocardiogram performed to assess fitness for AAA repair, revealed severe aortic valve stenosis (Aortic Valve Vmax=4.3cm/s, indexed orifice area <0.8cmsq), moderate LV impairment with moderate mitral regurgitation. Gated Cardiac CT showed heavily calcified tri-leaflet aortic valve. Invasive coronary angiography revealed complex 3-vessel Coronary Artery Disease (CAD). A multidisciplinary team comprising of operating vascular radiologist, cardiologist, vascular and cardiac surgeon and vascular anesthetist, concluded that the patient would be high risk for open cardiac surgery with impaired LV function, previous TIA and risk of intra-operative aneurysmal rupture. Therefore, TAVI was offered as the preferred treatment for the aortic stenosis and EVAR for the AAA.
Given the potential need for future intervention for coronary artery disease, an intra-annular sub-coronary TAVI device (Edwards Sapien 3-Edwards Life sciences Corp, Irvine, USA) was selected. This requires trans femoral access for implantation. There was concern that the required trans-femoral approach carried a risk of peri-operative aneurysm rupture. In addition, the newer valve delivery device with dynamic expansion mechanism would reach an equivalent of 24 French inner diameter post deployment which is a larger diameter than the 22 French device required to deliver the EVAR. A simultaneous TAVI and EVAR would avoid a re-puncture of the femoral artery with attendant operative risks for the patient. Therefore, a plan was formulated to undertake the TAVI under local anesthesia and if this was well tolerated to proceed with the EVAR immediately afterwards. Conversion to general anesthesia for the EVAR could be performed at that stage if necessary. Due to the narrow and angulated neck, an Aorfix device (Lombard Medical, Oxford, UK) was selected for the EVAR.
In a vascular hybrid suite, the patient was prepared and draped including access for an open surgical AAA repair if needed. Under local anesthesia, percutaneous vascular access was obtained, of bilateral femoral arteries and left brachial artery. A left femoral venous access was obtained for Temporary Pacing Wire (TPW) insertion. TAVI was performed in standard retrograde fashion; systemic heparinization (5000 IU) and balloon aortic valvuloplasty during rapid pacing (Figure 2). A balloon mounted Edwards Sapien 3 aortic valve was crimped on table and deployed successfully. There was trivial aortic regurgitation and a new left bundle branch block was noted with no further sequela. Hemodynamic benefit was immediate with reduction of trans-aortic gradient and reduction in left ventricular end diastolic pressure. Given a stable patient, the EVAR procedure was commenced (Figure 3a) under LA. Using fluoroscopic guidance, a bifurcated infrarenal abdominal aortic stent graft (Aorfix - Lombard) was deployed (Figure 3b). The main body was fixed and moulded to follow the neck angle. Procedure was completed with the limbs of the stent graft extended down to the iliac bifurcation bilaterally. The femoral arteriotomies were closed with prepositioned sutures (Pro Glide; Abbott Vascular, Santa Clara, CA, USA). There were no immediate post-operative complications.
The total procedure time was 160 min, the total fluoroscopic time was 80 min, and the amount of iodinated contrast medium (Visipaque 300 GE Amersham) used was 200 ml. The TPW was left in-situ for 24 hours and patient monitored on the coronary care unit for 24hours and then stepped down to a standard ward. The patient made an uneventful recovery and was discharged home from hospital on the second post-operative day.
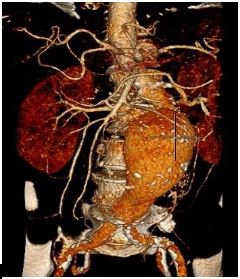
Figure 1: Volume rendered reformatted CT showing tortuous abdominal aorta aneurysm.
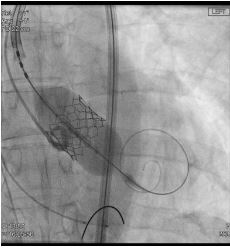
Figure 2: Angiogram showing deployment of balloon mounted aortic valve during a short period of high frequency RV pacing (180bpm).
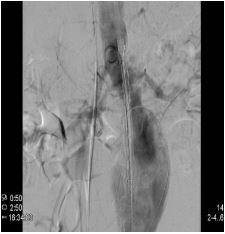
Figure 3a: Pre-EVAR angiographic image of the aortic aneurysm.
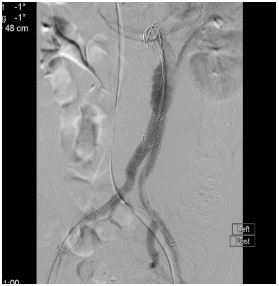
Figure 3b: Angiogram showing excellent final post-EVAR image with absence of end leak.
Discussion
Abdominal aortic aneurysm is a relatively common condition among elderly men with reported prevalence of 4.9% in the UK [2]. Large aneurysms have a natural history of progressive enlargement that can lead to rupture with a risk as high as 6.5% per year, in patients with aneurysmal diameter greater than 5cm [3,4]. Open surgical repair is generally associated with significant morbidity and mortality in the elderly population due to their multiple comorbidities as in the index patient. Increasing evidences showing EVAR has reduced short term operative mortality when compared to open surgery and has led to rising preference for EVAR in management of patients with AAA [5,6].
The Aorfix stent graft used in this case is a modular device composed of a circular ring body and helical limb nitinol frame covered by a woven polyester fabric [7]. It is extremely flexible and readily compliant with narrow tortuous vessels and several multicenter studies have demonstrated its utility in AAA patients unsuitable for repair with other available commercial grafts with Aorfix giving technical success as high as 96% [7].
In our institution TAVI procedures are usually performed via the Left Subclavian Artery (LSCA) with self-expanding valves in patients with poor femoral access or infra-aortic aneurysm. Trans-subclavian access for TAVI is usually an excellent alternative approach and in a prospective study showed lower rates of vascular complications at sheath insertion site and all bleeding events related to vascular complications. Subclavian access also reported a lower rate of acute kidney injury. Similar rates were observed for procedural success, major vascular complications and life-threatening bleeding events [8]. However in this case we preferred a Trans Femoral (TF) sub-coronary valve, allowing easier access if future coronary intervention were required. The LSCA approach was also felt to be unsuitable with concerns of the angulation affecting our ability to mount the valve on the balloon in-vivo. The LSCA approach is also ‘off-label’ and not recommended by device manufacturer.
After careful consideration and discussion with the patient we opted to perform both procedures under Local Anesthesia (LA) rather than General Anesthesia (GA). LA is known to improve safety and patient haemodynamic stability whilst reducing post-operative recovery, length of stay and pulmonary morbidity when compared to GA in patients undergoing either TAVI or EVAR procedures. As TF TAVI would involve traversing the angulated aneurysm with a 24 French sheath, this could be easily used to deploy the Aorfix EVAR main body. It was also felt the groin would be more hostile for an EVAR access later. More importantly the improved cardiac output post TAVI would increase the risk of rupture of the 8.3cm AAA.
We chose Local Anesthesia (LA) over General Anesthesia (GA) because of the known improved safety, simplicity, more stable patient hemodynamics, shorter post-operative recovery period, length of stay in high dependency units and reduction in pulmonary morbidity when compared to GA in patients who have undergone either TAVI and EVAR procedures [9]. While there is paucity of data on large scale studies of similar combined procedures, available reports suggests combined TAVI and EVAR procedures can reduce the risks associated with the perioperative period when compared to sequential procedures thereby improving post-operative mortality and morbidity [1]. As this was our first combined TAVI and EVAR, several precautionary steps were included, which may become less necessary with experience, reducing procedural time. Use of medical carbon dioxide as contrast agent for EVAR can decrease the iodinated contrast load especially in straight neck AAA. The Edward Sapiene Sheath with dynamic expansion mechanism required exchanging to a standard 24Fr sheath as its stiff bleed back prevention valve did not provide adequate seal to undertake EVAR. This case suggests that simultaneous TAVI and EVAR using a femoral approach under local anesthetic is feasible and may be considered. Given the complexity of the procedures and the numbers of personnel involved, clear communications and detailed planning between team members is essential for a successful outcome.
Acknowledgement
We would like to acknowledge Dr. Daniel Blackman, Dr. Michael Cross, Dr. Jeevan Mahaveer, Mr. Tom Wallace, Mrs. Anneke Winter (Aorfix Lombard proctor), Cardiac intervention, vascular intervention, vascular surgery and anesthetic support staff who helped and supported the case.
Compliance with Ethical Standards
On behalf of all authors, the corresponding author states that there is no conflict of interest. Authors declare no relationship with industry. The paper is not under consideration elsewhere; the paper's contents have been previously published and all authors have read and approved the manuscript. For this type of study formal consent is not required. Informed consent was obtained from all individual participants included in the study.
References:
- Sato Y, Horiuchi Y, Yahagi K, Okuno T, Kusuhara T, Yokozuka M, et al. Simultaneous transcatheter aortic valve implantation and endovascular aneurysm repair in a patient with very severe aortic stenosis with abdominal aortic aneurysm. J Cardiol Cases. 2018;17:123-125.
- Scott R. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomized controlled trial. The Lancet. 2002;360:1531-1519.
- Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999;230:289-296;296-297.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med. 2003;348:1895-1901.
- Greenhalgh RM, Brown LC, Kwong GPS, Powell JT, Thompson SG, EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet Lond Engl. 2004;364:843-848.
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535-1542.
- Weale AR, Balasubramaniam K, Macierewicz J, Hardman J, Horrocks M. Outcome and safety of AorfixTM stent graft in highly angulated necks - a prospective observational study (arbiter 2). Eur J VascEndovasc Surg Off J Eur Soc Vasc Surg. 2011;41:337-343.
- Petronio AS, De Carlo M, Bedogni F, Maisano F, Ettori F, Klugmann S, et al. 2-Year Results of CoreValve Implantation Through the Subclavian Access. J Am Coll Cardiol. 2012;60:502-507.
- Motloch LJ, Rottlaender D, Reda S, Larbig R, Bruns M, Müller-Ehmsen J, et al. Local versus general anesthesia for transfemoral aortic valve implantation. Clin Res Cardiol Off J Ger Card Soc. 2012;101:45-53.

