Nasal Chondromesenchymal Hamartoma in an Adult: A Rare Case Report
Bo-Nien Chen*
Department of Otolaryngology, Head and Neck Surgery, Hsinchu MacKay Memorial Hospital, Hsinchu, Taiwan
Received Date: 11/09/2020; Published Date: 08/10/2020
*Corresponding author: Bo-Nien Chen, Department of Otolaryngology-Head and Neck Surgery, Hsinchu MacKay Memorial Hospital, Hsinchu 30071, Taiwan. Tel: 88636119595; E-mail: chenbonien@yahoo.com.tw
Abstract
Nasal Chondromesenchymal Hamartoma (NCMH), first described by McDermott et al. in 1998, is an extremely rare benign tumor of the sinonasal tract that predominantly presents as a unilateral mass in infants and children. Only a few cases of NCMH in patients aged >16 years have been reported in English-language literature thus far. NCMH manifests a heterogeneous mixture of spindle cells, collagen fibers, and aberrant islands of chondroid and osseous tissue. Herein, we report the case of a 32-year-old woman with NCMH. The patient did not have local recurrence 2 years after endonasal endoscopic excision of the tumor. A brief review of the pathogenesis, clinical presentations, and histopathological characteristics of the NCMH was conducted. We presented a novel case of NCMH in an adult, which is extremely rare, but is amenable to surgical excision and demonstrates a favorable prognosis. Based on the relevance of NCMH and DICER1 syndrome, germline genetic testing for DICER1 is essential for the patients with NCMH and their family.
Keywords: Chondromesenchymal Hamartoma; Endoscopic Sinus Surgery
Introduction
Nasal chondromesenchymal hamartoma (NCMH) was first described by McDermott et al. in 1998 as an extremely rare benign tumor of the sinonasal tract that predominantly presents as a unilateral mass in infants and children [1,2]. NCMH is also called “chondroid hamartoma,” “mesenchymoma,” and “nasal hamartoma.” Morphologically, NCMH manifests a heterogeneous mixture of spindle cells, collagen fibers, and aberrant islands of chondroid and osseous tissue [3]. According to a 2018 review, only approximately 50 cases of NCMH have been reported in the English literature, with NCMH being reported primarily as a tumor in infants and children, with fewer than 10 cases reported in individuals aged >16 years [2]. Herein, we present an extremely rare case of NCMH in a 32-year-old woman, which was resected using the endonasal endoscopic approach, and provide a brief review of its pathogenesis, clinical presentations, histopathological characteristics, and management.
Case Report
A 32-year-old woman was referred to our hospital for a right nasal tumor, which had been discovered by a local medical doctor. She had a 3-month history of right nasal obstruction and denied other nasal symptoms. She did not have a history of nasal trauma or nasal surgery. She denied that she and her first and second-degree relatives had a history of cancer. On endoscopic nasal examination, a tumor was observed in the right nasal cavity. Computed Tomography (CT) of the sinonasal tract revealed a 0.6 × 2.2 × 2.7-cm polypoid tumor arising from medial side of the right middle turbinate with no evidence of bone destruction (Figure 1).
The patient subsequently underwent complete excision of the tumor using the endoscopic endonasal approach under endotracheal general anesthesia. Postoperative histopathology revealed that the tumor comprised chondroid and mesenchymal components. The chondroid component was composed of cellular cartilage foci with a hyaline cartilaginous matrix, whereas the mesenchymal component was represented by quite cellular zones consisting of plump fibroblast-like cells without any mitotic figures and atypia. All histologic features were compatible with those of NCMH (Figure 2).
No postoperative adjuvant treatment was administered, and the patient’s postoperative course was uneventful. After surgery, the patient’s symptoms improved. At the most recent follow-up, 2 years after surgery, no local recurrence was noted.
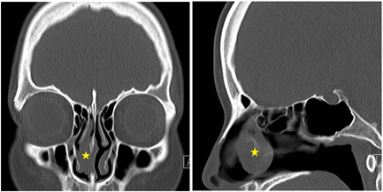
Figure 1: Preoperative coronal (A) and sagittal (B) CT images revealed a 0.6 × 2.2 × 2.7-cm3 polypoid tumor (asterisk) arising from the medial side of the right middle turbinate with no evidence of bone destruction.
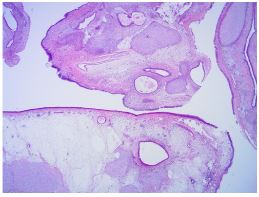
Figure 2A: Histopathological sections of the lesion. (A). On low magnification, the lesion revealed different histological patterns (hematoxylin and eosin [H&E], 10 × magnification).
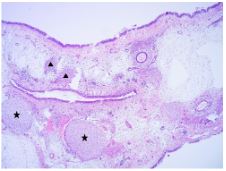
Figure 2B: The lesion contained chondroid (asterisks) and mesenchymal (triangles) components (H&E, 100 × magnification).
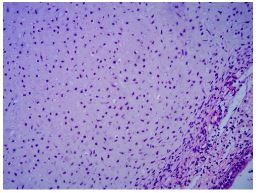
Figure 2C: Chondroid component at high magnification (H&E, 400 × magnification).

Figure 2D: Mesenchymal component at high magnification (H&E, 400 × magnification).
Discussion
Hamartomas are considered rare, benign lesions which can origin from different locations in the body. From a histological standpoint these formations can be epithelial, mesenchymal, or mixed. In the sinonasal tract, the following hamartomatous tumors are deemed to be on a spectrum and include respiratory epithelial adenomatoid hamartoma, chondro-osseous respiratory epithelial adenomatoid hamartoma, seromucinous hamartoma, olfactory epithelial hamartoma, and NCMH [3]. Morphologically, NCMH manifests a heterogeneous mixture of spindle cells, collagen fibers, and aberrant islands of chondroid and osseous tissue. NCMH is considered an upper airway analog of mesenchymal hamartoma of the chest wall [4]. NCMH is a rare benign tumor of the sinonasal tract, typically presenting in infants and children aged <1 year. Most patients are male, with a male-to-female ratio of 2.2:1 [5]. However, we herein present an extremely rare case in a 32-year-old woman. Only a few cases of NCMH in patients aged >16 years have been reported in English-language literature thus far. The oldest patient with NCMH reported in the literature was aged 70 years [2]. In addition to the sinonasal tract, NCMH has been reported in the oropharynx, orbit, lacrimal sac, ectopic thyroid tissue, and bronchus [5-9].
NCMH pathogenesis remains unclear. Because a vast majority of the NCMH cases were diagnosed during the newborn period, with much fewer cases diagnosed during the adolescence period, NCMH was initially believed to have a developmental or congenital origin [3,10]. However, later adult case reports refuted this hypothesis because the adult patients concerned had no symptoms during their childhood. Hormonal and environmental factors with an underlying genetic predisposition have also been considered in its etiopathogenesis [4,10]: in adults, NCMH may etiologically represent a tissue response to an irritant, such as chronic sinusitis, rather than represent an inborn germline mistake [2]. However, no clear pathogenesis has been described; therefore, defining whether NCMH is a primary or secondary event to a chronic inflammatory course is difficult [3].
Our case presented with a 3-month history of right nasal obstruction and denied other nasal symptoms. Clinical presentations of patients with NCMH vary in terms of lesion size and localization. NCMH presents usually with sleep-related breathing disorder due to nasal obstruction, feeding difficulties in infants, recurrent rhinosinusitis, middle ear effusion, epistaxis and watery rhinorrhea, hyposmia, or frontal headache with no nasal symptoms. Moreover, intraorbital and intracranial extension may lead to oculomotor disorders, or exophthalmos as well as neurologic deficits [3,8,10,11]. The case of a patient presenting with intraoral symptoms due to the invasion of the oral cavity has also been reported [5]. Despite being a benign lesion, NCMH can exhibit necrosis and local destruction. The lesions can appear aggressive on imaging, extending into bony structures, including the cranium and/or the orbital cavity, which can masquerade as malignant tumors [2,5,11]. In approximately 20% of NCMH cases, the tumor extends into the anterior cranial fossa [11]. Differential diagnosis includes hemangioma, angiofibroma, antrochoanal polyp, nasoethmoidal encephalocele, meningoencephalocele, nasal glioma, rhabdomyosarcoma, lymphoma, and chondrosarcoma [3,12]. Considering NCMH in the differential diagnosis of sinonasal tumors is critical in adult patients to avoid misdiagnosis [2].
Before surgical excision of NCMH, a detailed CT or preferably Magnetic Resonance Imaging (MRI) should be performed [2]. MRI facilitates better tissue characterization and delineation of involvement of adjacent structures than does CT. On T1-weighted MRI images, NCMH demonstrates as heterogeneous lesions with low signal intensity, whereas it exhibits high signal intensity on T2-weighted MRI images with significantly heterogeneous contrast enhancement. On CT images, NCMH appears as nonencapsulated, poorly defined heterogeneous soft-tissue lesions with mostly solid and cystic components [3,5,12]. In one case, CT images revealed NCMH as a calcification due to osseous components presented in the lesion [4]. Thus, calcification may be a crucial diagnostic clue, which may help differentiate NCMH from other nasal tumors [12]. Because NCMH has aggressive and infiltrative features, bony erosions often simulate malignant tumors on imaging [4,12]. An accurate diagnosis is crucial to avoid unnecessary adjuvant therapy [12].
Because of its behavior typical to benign tumors, the complete surgical excision is the treatment of choice for NCMH, and it demonstrates favorable results generally. The endoscopic endonasal approach is the standard of surgical treatment [3-5,11]. In our case report, an excellent prognosis is achieved by complete excision using the endoscopic endonasal approach under general anesthesia. However, the infiltrative nature of NCMH can make it difficult to obtain a clean surgical margin, particularly in patients with intracranial invasion [4,11]. In case of incomplete excision, close follow-up MRI should be recommended. Nevertheless, some data indicate that even complete excision cannot entirely eliminate the risk of NCMH recurrence [3].
A definitive diagnosis is primarily through histopathological examination. The microscopic picture of NCMH presents as irregular islands of mature as well as immature hyaline cartilage with occasional chondrocytes. The cartilage islands have well-demarcated boundaries with the surrounding stromal tissues, which have a myxoid background. No atypical mitotic figures or malignant characteristics are usually observed. A study reported spindle cells with occasional mitotic figures [3]. Immunohistochemistry results demonstrated that the tumor cells are positive for CD34, vimentin, S100, EMA, and GFAP [4]. Although NCMH is a benign lesion, the first and exclusively reported adult case of its malignant transformation was described in 2013 [13]. Hence, in NCMH cases, particularly adult cases, the possibility of malignant transformation should be considered and thus comprehensive histologic examination should be performed [4].
The relevance of NCMH and Pleuropulmonary Blastoma (PPB) with DICER1 mutation and various associated entities has been reported. DICER1 is an RNAse endoribonuclease, crucial in the microRNA and RNA-interference functional pathways [4,14]. Pathogenic germline DICER1 variants cause an autosomal dominant cancer predisposition syndrome with various manifestations [14]. DICER1 syndrome mostly affects children and young adults and features a unique constellation of benign and malignant neoplastic and dysplastic lesions. PPB is the hallmark tumor of DICER1 syndrome. Other manifestations include ovarian sex cord-stromal tumors (particularly Sertoli-Leydig cell tumor), lung cysts, cystic nephroma, renal sarcoma and Wilms’ tumor, nodular hyperplasia of the thyroid, thyroid carcinoma, NCMH, ciliary body medulloepithelioma, genitourinary embryonal rhabdomyosarcoma, and brain tumors (including pineoblastoma and pituitary blastoma) [4,14-15]. Clinicians should therefore be conscious of these disease associations and of the possibilty of NCMH in patients presenting with sinonasal symptoms and a history of any of the aforementioned manifestations [5]. DICER1 syndrome is generally caused by heterozygous germline loss-of-function DICER1 alterations accompanied on the other allele by somatic missense mutations occurring at one of few mutation hotspots within the sequence encoding the RNase IIIb domain [15]. Stewart et al. observed pathogenic germline DICER1 mutations in 75% of patients with NCMH and PPB [16]. Vasta et al. described 38% of NCMH cases had at least one additional DICER1-associated tumor [14]. For patients in which NCMH is diagnosed, germline genetic testing for DICER1 is essential for the patient and their family [14]. However, we did not offer DICER1 germline testing for this patient because our hospital does not have the equipment for this examination.
Conclusion
In conclusion, we presented a novel case of NCMH in an adult and provided a review of the associated main clinicopathological features. NCMH presents rarely; therefore, physicians should be aware of this entity and consider NCMH in the differential diagnosis of sinonasal masses in adult patients. Endoscopic endonasal excision is the standard of surgical management, and excellent prognosis is achieved. Based on the relevance of NCMH and DICER1 syndrome, germline genetic testing for DICER1 is essential for the patients with NCMH and their family.
Declaration of Conflicting of Interests
None
References:
- McDermott MB, Ponder TB, Dehner LP. Nasal chondromesenchymal hamartoma: an upper respiratory tract analogue of the chest wall mesenchymal hamartoma. Am J Surg Pathol. 1998;22:425-433.
- Mirchia K and Naous R. Nasal chondromesenchymal hamartoma: rare case report in an elderly patient and brief review of literature. Case Rep Pathol. 2018;2018:5971786.
- Golbin DA, Ektova AP, Demin MO, Lasunin N, Cherekaev VA. Nasal chondromesenchymal hamartoma with skull base and orbital involvement: case presentation. Cureus. 2018;10:e2892.
- Unal A, Kum RO, Avci Y, Unal DT. Nasal chondromesenchymal hamartoma, a rare pediatric tumor: case report. Turk J Pediatr. 2016;58:208-211.
- Mason KA, Navaratnam A, Theodorakopoulou E, Chokkalingam PG. Nasal chondromesenchymal hamartoma (NCMH): a systematic review of the literature with a new case report. J Otolaryngol Head Neck Surg. 2015;44:28.
- Gunduz K, Kurt RA, Kaygusuz G, Kankaya D. Primary chondromesenchymal hamartoma of the orbit. Ophthalmic Plast Reconstr Surg. 2009;25:324-327.
- Li GY, Fan B, Jiao YY. Endonasal endoscopy for removing nasal chondromesenchymal hamartoma extending from the lacrimal sac region. Can J Ophthalmol. 2013;48:e22-e23.
- Szepesi A, Juhasz Z, Kover A, Kajtar B, Benedek N, Kalman E, et al. Chondromesenchymal hamartoma in ectopic thyroid tissue in a neonate. European J Pediatr Surg Rep 2019;7:e39-e42.
- Sardon O, Maehuenda C, Santiago M, Toran N, Korta J, Corcuera P, et al. Endobronchial chondromesenchymal hamartoma. An Pediatr (Barc). 2010;72:263-266.
- Kim JE, Kim HJ, Kim JH, Ko YH, Chung SK. Nasal chondromesenchymal hamartoma: CT and MR imaging findings. Korean J Radiol. 2009;10:416-419.
- Nakaya M, Yoshihara S, Yoshitomi A, Baba S. Endoscopic endonasal excision of nasal chondromesenchymal hamartoma with intracranial extension. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:423-425.
- Wang T, Li W, Wu X, Li Q, Cui Y, Chu C, et al. Nasal chondromesenchymal hamartoma in young children: CT and MRI findings and review of the literature. World J Surg Oncol. 2014;12:257.
- Li Y, Yang QX, Tian XT, Li B, Li Z. Malignant transformation of nasal chondromesenchymal hamartoma in adult: a case report and review of the literature. Histol Histopathol. 2013;28:337-344.
- Vasta LM, Nichols A, Harney LA, Best AF, Carr AG, Harris AK, et al. Nasal chondromesenchymal hamartomas in a cohort with pathogenic germline variation in DICER1. Rhinol Online. 2020;3:15-24.
- de Kock L, Priest JR, Foulkes WD, Alexandrescu S. An update on the central nervous system manifestations of DICER1 syndrome. Acta Neuropathol. 2019.
- Stewart DR, Messinger Y, Williams GM, Yang J, Field A, Schultz KAP, et al. Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder. Hum Genet. 2014;133:1443-1450.

