Novel Perspectives for Treating and Preventing Dementia by Complementary Medicine
Frank Comhaire*
Department of Internal Medicine, Endocrinology and Metabolic Diseases, University Hospital, Ghent, Belgium
Received Date: 17/08/2020; Published Date: 02/09/2020
*Corresponding author: Department of Internal Medicine, Endocrinology and Metabolic Diseases, consulting specialist at Fertility Belgium clinic, Weststraat, 16, 9880, Aalter, Belgium. Tel: 0032 475 618 555; Email: frank@comhaire.com
Abstract
The medical case report is described of two patients suffering from mild to moderate dementia in whom the progression of the disease was successfully halted by complementary phytotherapy and nutraceutical administration. Treatment of insulin resistance by natural plant extracts, and correction of disordered immunity was of pivotal importance. The potential of food supplementation with vitamins, plant- and mushroom extracts, and essential fatty acids in the prevention and treatment of dementia, Alzheimer’s disease in particular, is substantiated by the published results of experimental, epidemiological, pragmatic, controlled, and observational studies. It is concluded that early detection of incipient dementia and treatment with judiciously composed complementary nutraceuticals may reduce the risk of progression of neurodegenerative diseases.
Keywords: Dementia; Alzheimer’s Disease; Nutraceutical; Phytotherapy; Food Supplementation
Introduction
Dementia is the worst disease to affect mostly elderly people, since it does not kill, but it removes a pivotal aspect of personality, being the cognitive capacities and memory. The most common cause of dementia is Alzheimer’s disease (AD) which is due to the deposit of tau protein and amyloid plaques in the grey matter of the brain. Aside from genetic predisposition, external factors such as nutrition and lifestyle are important through influencing epigenetics. It would be simplistic to consider and treat dementia as an exclusive neurologic brain disease since vascular, immunologic, endocrinologic and metabolic deregulation are involved it its pathogenesis.
In the present paper describes two exemplary cases of AD in elderly, in whom the evolution to further neurodegeneration could be halted by treatment using primarily nutraceutical food supplementation. Opportunities for preventing dementia based on complementary medicine are discussed.
Medical Case Description
This 77-year-old male patient was referred to the consultation in January 2019because of impaired memory, degenerating progressively and rather rapidly during the preceding years. Five years before consultation his biomarker profile was negative, with lumbar fluid testing being completely normal, suggesting the patient did not suffer from AD. The diagnosis was given of “frontotemporal dementia”, with a family background of neurodegenerative disease. Treatment with the acetylcholinesterase inhibitor Donezepil10 mg per day plus Quetiapine was prescribed. In addition, the patient took Rosuvastatine because of an elevated serum cholesterol concentration.
Clinical propaedeutic examination upon intake revealed an excellent physical condition with bloodpressure of 135/85 and slow heart rate of 52/min. There were no signs of cardiovascular disease. Duplex Doppler examination of the carotid arteries was perfectly normal, without signs of arteriosclerosis. The electrocardiogram was normal presenting no characteristics of coronary ischemia, nor ventricular hypertrophy. Blood analysis revealed several abnormalities related to both cellular and humoral immunity, with low number of CD3 T-lymphocytes (643, normal range 700-20100) and elevated number of Natural Killer cells (34.6, normal range 7-31). There was an increased concentration of Immunoglobulin IgG4 (2.88 g/L, normal range 0.03-2.01), of IgA (5.44g/L; normal range 0.70-4.00), anda borderline positive titre of Antinuclear Antibodies (ANA 1/80).
NeuroSpect scanning was performed before and after 4 months of treatment.
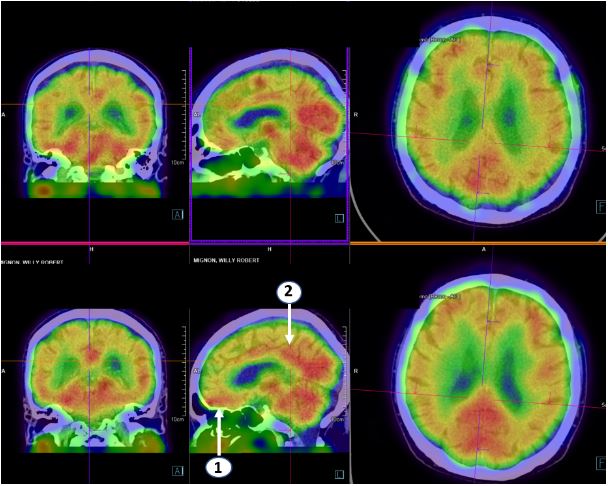
Figure 1: NeuroSpect scan of patient W.M.with registration before (top pannels) and after (bottom pannel) 4 months of treatment. Treatment resulted in increased isotope activity in the orbito-frontal region (1,Brodmann area11) and in the anterior cingular region (2.Brodmann area’s 31 and 32).(Courtesy Bieke Van Den Bossche MD. PhD. Maria Middelares hospital, Ghent, Belgium)
The diagnosis of neurodegenerative disease was accepted with characteristics of (auto)-immune disorder. Treatment was initiated with intravenous infusions of methylprednisolone 125 mg, injected over a 2-hour period, and repeated every 2 weeks for 2 months. After 2 months the frequency of infusion therapy was decreased to once per month.Patient was given complementary treatment with: fermented red rice to replace the statin, Meldonium (Mildronate®, Grindeks, Vilnius, Latvia)1000 mg per day, to increase glucose uptake into the mitochondria and to improve cerebral blood supply, Hericium erinaceus extract, the nutraceutical QALY® (see annex), and Vitamin D. Treatment stabilised the memory function, and increased awareness and cognitive functions, which was confirmed after 15 months. The concentrations of IgG4and IgA decreased, and the repeat NeuroSpect scanning revealed enhanced isotope activity in the orbito-frontal and the anterior cingular regions.
Case V.H.C.
This76 years old female patient was diagnosed with AD 5 years before consultation, initially presenting impaired short-term memory and MMSE of 23/30. She was treated by a neurologist with Donepezil, which she did not tolerate well, and which was replaced by Memantine.When the patient was seen at consultation 4 years ago, her condition haddeteriorated further. At that time she took Selenium, vitamins A, C, E and D, Rosuvastatin, Bisoprolol and acetylsalicylic acid. Propaedeutic physical examination did not reveal any abnormalities, with blood pressure of 120/80. Echography of the liver and duplex Doppler examination of the carotid arteries were normal.
Blood analyses revealed an elevated postprandial concentrations of C-peptide (80.4ng/mL; normal range 1.10-4.40)and of insulin (56 µU/mL; normal range: 2.6-24.9) indicating insulin resistance (figs 2 and 3). The anti-nuclear antibody test was borderline positive with titre of 1/80. Endocrine assessment was normal regarding thyroid and adrenal functions. Because of the well-known adverse effect of Rosuvastatin in inducing insulin resistance and (pre)-diabetes, this medicine was replaced by fermented red rice. Patient was given capsules containing 400mg of the extract (8:1) of Momordica charantia (bitter melon) together with alfa lipoid acid, as well as the composed neutraceutical QALY® (see annex). Meldonium was administered in a dose of 1000 mg per day. Hypertension was maintained under control by the combination of Olmesartan and Amlodipine (Sevicar® 20/5, Daichi Sankyo). Because of agitation, Sulpiride was given to replace Memantine.
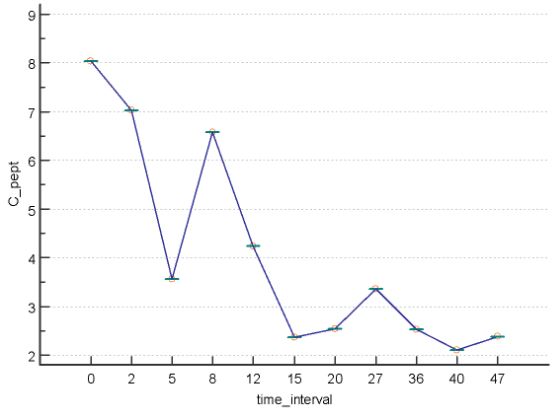
Figure 2: Postprandial concentration of c-peptide (on the vertical axis, in ng/mL) at different time intervals (on the horizontal axis, in months) before and after initiation of treatment with Momordica charantia extract combined with alfa lipoic acid.In month6 patient interrupted treatment because of a surgical intervention. She resumed treatment in month 11.
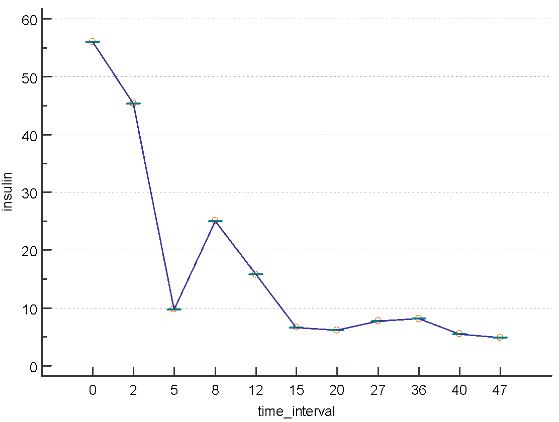
Figure 3: Postprandial insulin concentration (on the vertical axis, in µU/mL) at different time intervals (on the horizontal axis, in months) after initiation of treatment (legend see figure 2).
During 4 years of follow-up the mental and physical condition of the patient remained stable, with normal insulin sensitivity, and normal postprandial concentrations of C-peptide (fig 2) and of insulin (fig 3). As expected, the latter concentrations were mutually correlated (r=0.96, P<0.0001).
Discussion of the Cases
Neurodegenerative disease was mild to moderate in both patients described, with predominant short-term memory deficit. Their disease was progressive with increasing cognitive impairment, but conventional treatments failed to halt, let alone to improve, their condition.
In patient W.M. the immune disorder was considered of pivotal importance for the progression of the disease. Intermittent high dose intravenous cortico-therapy aimed at reducing the impact of autoimmunity on the cerebral microvascular blood supply, on the metabolic function of the neurons, and on signal transmission through the dendrites. This approach avoids systemic and cerebral adverse effects of continuous oral immunosuppressive corticoid administration, since physiological feedback regulation of the hypothalamo-pituitary-adrenal (HPA)-axis is restored within 48 to 72 hours after the intravenous administration. Treatment with the extract of the fungus Hericium erinaceus and with Meldonium enhanced the benefits of immunosuppression, acting through complementary pathways. In patient V.H.C. treatment focussed on the metabolic aspects, especially insulin resistance, probably caused by the long-term intake of Rosuvastatin. The resulting hyperinsulinism has been documented to accelerate the neurodegenerative process. Red yeast rice lowered the cholesterol concentration and increased the proportion of high density lipoproteins, without impairing insulin sensitivity, thanks to the synergistic effect of several natural constituents. Momordica charantia extract and alfa lipoic acid increased the cellular response to insulin,improving glucose metabolism. Both patients were administered Meldonium (Mildronate®, Grindeks, Vilnius, Latvia) that inhibits the uptake of fatty acids into the mitochondria by competing with acetyl-carnitines, thus enhancing glucose entry and aerobic metabolism. They also took the nutraceutical food supplement QALY® because of its judicious composition with vitamins, plant extracts and minerals, as discussed below.
General Considerations
So far no efficacious treatment has been available for the treatment of AD. Recent publications highlight the potential of life style and nutritional measures to reduce the prevalence of this disease.Several clinical and biomarkers have been described [1], and measurement of plasma pTau181 [2] was found to correlate with cortical tau protein deposition assessed by PET scan [3], which may provide an opportunity for diagnosing the diseaseand initiating therapeutic intervention at an early stage of neurodegeneration.
According to the Lancet Commission on Dementia [4]12 risk factors for dementia should be considered, namely: less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, traumatic brain injury, and air pollution. It is the conviction of the author that stress, be it moderate or severe but long lasting, should be added to the list of factors that can be modified by timely intervention. Recent research highlights the pivotal role of nutrition, in particular the intake of flavonoids [5]. Furthermore autoimmune diseases [6-8], such as inflammatory bowel disease [9], were found to be associated with increased risk of AD.
The unifying mechanisms involved with many of these predisposing factors are immune disorder, chronic inflammation, oxidative stress and neuro-endocrine deregulation, probably inducing genetic and epigenetic alterations. Complementary treatments may possibly correct these alterations.
Cerebral blood supply can be improved by several phyto therapeutic agents. While serum lipid concentrations can favourably be influenced by the intake of fermented red rice [10],capillary blood supply is enhanced by the extract of Ginkgo biloba that exerts a anti-thrombotic action and improves brain cognitive function [11].
Meldonium acts as an inhibitor of acetyl-carnitine synthesis and reduces the transfer of fatty acids into the mitochondria. This promotes the compensatory glucose-pyruvate use in the aerobic metabolism of the Krebs cycle. Meldonium decreases fatty acid accumulation in the endothelial cells of the arteries, and enhances cerebral blood supply with resulting increased cellular metabolism [12,13].
Glucose metabolism in the cerebral neurones is exceptionally important,but is impaired in case of insulin resistance. The latter should always be detected in patients with dementia, and may related to the long-term use of statins [14].Conventional treatment uses Metformin, but this substance can be ketogenic, and its dose should be adapted depending on renal function. Metformin acts on the molecular pathway enhancing the adult neural precursor proliferation/self-renewal and differentiation, mainly by activating the AMPK pathway [15]. The extract of Momordica charantia (bitter melon) is non-toxic and highly efficient in restoring insulin sensitivity [16]. The triterpenoids of bitter melon induce activation of AMPK [17,18] with equal beneficial effect on the generation of neural precursor cells.Its effectiveness persists during long-term intake, and the substance is well tolerated.The efficiency of Momordica extract can be enhanced by combining it with alpha lipoic acid[19], thus improving neuronal function.
Sodium dichloroacetate is an inhibitor of mitochondrial pyruvate dehydrogenase kinase and boosts aerobic metabolism and the generation of adenosine triphosphate (ATP). Its efficacy has been proven for the treatment of brain dysfunction in patients with myalgic encephalomyelitis [20].Recent studies suggest this substance may alleviate metabolic impairment in the neurons of patients with AD [21] and stimulate angiogenesis [22].
Neuronal function is also enhanced by supplementingvitamins B9 (folic acid) and B12(methylcobalamine) which reduce homocysteine concentration [23], and prevent thinning of the cerebral cortex in persons with elevated serum homocysteine concentration. Increased metabolic energy production in the neurons will enhance signal transduction through the axons. This can be improved further by supporting the function of the Schwann cellsthrough the supplementary administration of phosphatidylcholine [24] which will be incorporated into the myelin sheath.
The pivotal importance of inflammation in the pathogenesis of dementia cannot be overemphasised[25,26]. Non-steroidal anti-inflammatory drugs (NSAIDs) may prevent AD, though the evidence from observational studies must be interpreted with restraint [27]. Anthocyanidins are a subclass of flavonoids with important health benefits [28] thanks to their potent anti-inflammatory and anti-oxidant capacities.Pine bark extract is a rich source of anthocyanidins [29].This extract was found to decelerate plaque development [30] and to attenuate amyloid-βand Tau misfolding [31], improving memory in experimental AD in miceas well as in humans [32]. Indeed, elevated nutritional flavonoid intake was related to an important decreased risk of developing AD [5].
Unhealthy lifestyle, inadequate nutrition with excess intake of ultra-processed food, insulin resistance, impaired arterialblood supply and hypertension, exposure to environmental toxins, and long-lasting stress all result in oxidative stress, which is considered an important issue in understanding the pathogenesis of AD [33,34].Hence,antioxidant treatment may help to prevent and to treat neurodegenerative diseases [35-37].Plant extracts with antioxidant properties are probably most appropriate for this purpose [38,39,5]. Astaxanthin,that is present in the biomass of the microscopic algae Haematococcus pluvialis, is a strong carotenoidantioxidant freeof toxicity. Based on its antioxidative, anti-inflammatory and anti-apoptotic properties, Astaxanthin can tackle neurodegeneration [40].Thanks to its antioxidative effect at the level of the mitochondria, the oxidoreductase Ubiquinol Q10does improve aerobic metabolism and energy generation of Krebs cycle. The neuroprotective potential related to phytochemicals [41] in the extract of Rhodiola rosea has been successfully applied in the therapeutic strategy of AD[42], improving learning and memory functions [43]. This phyto-adaptogen also reduces general anxiety and depression that are commonly associated with incipient dementia[44].
Similarly, edible medical mushroom extracts may be administered for the management of neuro-degenerative diseases [45,46] and depression [47]. The extract of Hericium erinaceus was proven particularly effective in this respect [48,49]. Poly-unsaturated omega-3 fatty acids, the long chain eicosapentaenoid acid (EPA) and docosahexaenoid acid (DHA) in particular, upregulate gene expression concerned with neurogenesis, neurotransmitters and connectivity, improving endothelial nitric oxide generation, enhancing brain acetylcholine levels, and protecting neurons from inflammation caused by cytokines and prostaglandins [50]. These fatty acids are abundant in fish oil, but krill oil may be preferable for of ecological reasons, and because EPA and DHA are present as phospholipids which enhances their cellular uptake [51].
Conclusions
The pathogenic mechanisms acting during the development of dementia in general, and of AD in particular, are multiple and only partially understood. It is simplistic to consider and treat this disease as if it were exclusive involving the brain. Pragmatic and observational studies have revealed that several of the pathogenic mechanisms can positively be influenced by the administration of food supplements and/or phyto-therapeutic agents. These can be combined in order to synergistically enhance their therapeutic potential. Such combined nutraceuticals should be administered either prophylactically, or at the onset of signs or symptoms of dementia. Adaptation of live style, exercising, healthy nutrition, and the detection and treatment of possible underlying systemic diseases must be promoted, but insulin resistance, chronic inflammation, and oxidative stress should also be corrected. In addition, neuroprotection should be offered by supplementary intake of particular vitamins, minerals, plant and mushroom extracts, as well as essential fatty acids. Two patient cases are described illustrating the successful implementation of this strategy.
Acknowledgments
The author expresses his gratitude to pharmacist Johan Vandaele of JonaPharmaLtd for preparing the food supplements and for technical advice.
Competing Interests
The author holds the patent of the formulation of QALY1®.
Grant Information
This research has not received any external financial support or grant.
Annex
Formulation of the nutraceutical QALY1+2® (Jona Pharma, Elversele-Temse, Belgium):
Qaly 1:
Astaxanthin (biomass of Haematococcus pluvialis):2 mg;
Oxido-reductase Ubiquinone Q10: 25 mg;
Extract of pine bark (Pinus maritima): 21 mg;
Extract of rosenroot(Rhodiola rosea): 175 mg;
Vitamin B9 (Folic acid): 0.2 mg;
Vitamin B12 (Methylcobalamine): 1.25 µg;
Selenomethionine: 14 mg;
Zinc bis glycinate: 3mg Zn.
For one coated tablet.
Qaly 2:
Krill oil (EPA: 250 mg, DHA: 250 mg).
For one capsule
One tablet of QALY 1 should be taken together with one capsule of QALY 2, once or twice per day, depending on the prescription of the doctor or the nutritionist.
References:
- Fortea J, Vilaplana F, Carmona-Iragui M et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. The Lancet 2020; 395: 1988-1997. DOI: 10.1016/S0140-6736(20)30689-9.
- Thijssen E, La Joie R, Wolf AZ, et al.Diagnostic value of plasma tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020; 26: 387-397. DOI: 10.1038/s41591-020-0762-2
- La Joie R, Visani AV, Baker SL et al. Prospective longitudinal atrophy in Alzheimzer’s disease correlates with the intensity and topography of baseline tau-PET. Science translational medicine 2020; 12, eaau 5732. Doi 10.1126scitranlmed aau5732
- Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 2020; 396: 291-360. DOI: 10.1016/S0140-6736(17)31363-6
- Shishtar E, Rogers GT, Blumberg JB et al. Long-term flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham offspring cohort.Am J Clin Nutr. 2020;112: 343-353. DOI: 10.1093/ajcn/nqaa079.
- McKeon A. Autoimmune encephalomyelitis and dementias. Continuum (Minneap Minn) 2016; 538-558. DOI: 10.1212/CON.0000000000000299.
- Wotton CJ, Goldacre MJ. Association between specific immune diseases and subsequent dementia: retrospective record-linkage cohort study, UK. J Epidemiol Community Health 2017; 71: 576-583. DOI: 10.1136/jech-2016-207809.
- Li X, Sundquist J, Zoller B, Sundquist K. Dementia and Alzheimer’s disease risks in patients with autoimmune disorders. Geriatr Gerontol Int 2018; 18: 1350-1355. DOI: 10.1111/ggi.13488.
- Zhang B, Wang HE, Bai YM, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut 2020; 0: 1-7. DOI: 10.1136/gutjnl-2020-320780.
- Comhaire FH. A comprehensive nutriceutical approach to metabolic and vascular health. Journal of endocrinology and diabetes 2016; 3(1): 1-4; DOI: 10.15226/2374-6890/3/4/00157
- Silberstein RB, Pipingas A, Song DA, et al. Examining brain-cognitive effects of Ginko biloba extract: brain activation in the left temporal and left prefrontal cortex in an object working memory task. Evid based complement alternat med 2011; 164139. DOI: 10.1155/2011/164139.
- Sjakste N, Gutcaits A, Kalvinsh I. Mildronate: an antiischemic drug for neurological indications. CNS Drug Rev 2005; 1: 151-168. DOI: 10.1111/j.1527-3458.2005.tb00267.x.
- Berlato DG, Valle de Bairros A. Meldonium: pharmacological, toxicological, and analytical aspects. Toxicology Res and Application 2020. DOI: 10.1177/2397847320915143.
- Mitchell P, Marette A. Statin-induced insulin resistance through inflammasome activation: sailing between Scylla and Charybdis. Diabetes 2014; 63: 3569-3571. DOI: 10.2337/db14-1059.
- Fatt M, Hsu K, He l, et al. Metformin acts a two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports 2015; 5: 988-995. DOI: 10.1016/j.stemcr.2015.10.014.
- Comhaire F. Nutriceutical approach to the metabolic syndrome. Endocrinology &Metabolic Syndrome 2014; 3 (3): 1-4. Doi.org/10.4172/2161-1017.1000134.
- Iseli TJ, Turner N, Zeng XY, et al. Activation of AMPK by bitter melon triterpenoids involves CaMKK.PLoS One2013; 25: 8(4): e62309. DOI: 10.1371/journal.pone.0062309.
- Jia S, Shen M, Zhang F, Xie J. recent advances in Momordica charantia: functional components and biological activities. Int J Mol Sci. 2017; 18: 2555.DOI: 10.3390/ijms18122555
- Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol 2011; 2: 69. DOI:10.3389/fphar.2011.00069.
- Comhaire. Treating patients suffering from myalgic encephalopathy/chronic fatigue syndrome (ME/CFS) with sodium dichloroacetate: An open-label, proof–of-principle pilot trial. Med Hypotheses 2018; 114: 45-48. DOI: 10.1016/j.mehy.2018.03.002.
- Patel V, Zhang X, Tautiva NA, et al. Small molecules and Alzheimer’s disease: misfolding, metabolism and imaging. Curr Alzheimer Res 2015; 12: 445-461. DOI: 10.2174/1567205012666150504145646.
- Zhao H, Mao J, Yuan Y, et al. Sodium dichloroacetate stimulates angiogenesis by improving endothelial precursor cell function in an AKT/GSK-3β/Nrf2 dependent pathway in vascular dementia rats. Front Pharmacol 2019; 10: 523.DOI: 10.3389/fphar.2019.00523.
- Comhaire F, Decleer W. Can the biological mechanisms of ageing be corrected by food supplementation? The concept of health care over sick care. Aging Male. 2020; 1-12. DOI: 10.1080/13685538.2020.1713080.
- Ylilauri MPT, Voutilainen S, Lönroos E, et al. Association of dietary choline intake with risk of incident dementia and with cognitive performance: the Kuopio ischemic heart disease risk factor study. Am J Clin Nutr 2019; 110: 1416-1423; DOI: 10.1093/ajcn/nqz148.
- Rogers J, Webster S, Kue KF et al. Inflammation and Alzheimer’s disease pathogenesis. Neurobiol Aging 1996; 17: 681-686. DOI: 10.1016/0197-4580(96)00115-7.
- Kinney JW, Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (NY) 2018; 4: 575-590. DOI: 101016/j.trci.2018.06.014.
- Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiologic evidence. CNS Neurol Disord Drug Targets. 2010; 9: 132-139. DOI: 10.2174/187152710791012026.
- Li D, Wang P, Luo Y, et al. Health benefit of anthocyanins and molecular mechanisms: Update from recent decade. Crit Rev Food Nutri 2017; 24: 1729-1741. DOI: 10.1080/10408398.2015.1030064.
- Iravani S, Zolfaghani B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci 2011; 6: 1-11.
- Paarmann K, Prakash SR, Krohn M, et al. French maritime pine bark treatment decelerates plaque development and improves spatial memory in Alzheimer’s disease mice. Phyomedicine 2019; 57: 39-48. DOI: 10.1016/j.phymed.2018.11.033.
- Ono K, Zhao D, Wu Q, et al. Pine bark polyphenolic extract attenuates amyloid- and tau misfoldingin a model system of Alzheimer’s disease neuropathology. J Alzheimers Dis 2020; 73: 1597-1606. DOI: 10.3233/JAD-190543.
- Orhan IE, Daglia M, Nabavi SF, et al. Flavonoids and dementia: an update. Curr Med Chem 2015; 22: 1004-1015. DOI: 10.2174/0929867322666141212122352.
- Smith MA, Rottkamp CA, Nunomura A, et al. Oxidative stress in Alzheimer’s disease. Biochem Biophys Acta 2000; 1502: 139-144. DOI: 10.1016/S0925-4439(00)00040-S.
- Huang WJ, Zhang XIA, Chen WW. Role of oxidative stress in Alzheimer’s disease. Diomd Rep 2016; 4: 519-522. DOI: 10.3892/br.2016.630.
- Di Mateo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr Drug Targets 2003; 2: 98-107. DOI: 10.2174/092986710791859324.
- Gilgun-Sherki Y, Melamed E, Offen D. Antioxidant treatment in Alzheimer’s disease: current state. J Molec Neurosci 2003; 21: 1-11. DOI: 10.1385/JMN:21:1:1.
- Farina N, Liewellyn D, El Kareem Nasr Isaac M, Tabet N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev 2017; 18:4. DOI: 10.1002/14651858.CD002854pub5.
- Pohl F, Lin PKT. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018; 23: 3283; DOI: 10.3390/molecules23123283.
- Roy A. Role of medicinal plants against Alzheimer’s disease. Int J Complement Alt Med 2018; 11: 205-208. DOI: 10.15406/ijcam.2018.11.00398.
- Fakhri S, Aneva IY, Farzaei MH, Sobarzo-Sanchez E. The neuroprotective effects of Astaxanthin: therapeutic targets and clinical perspective. Molecules 2019; 24: 2640. DOI: 10.3390/molecules24142640.
- Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev 2012; 6: 81-90. DOI: 104103/0973-7847.99898.
- Nabavl SF, Braiidy N, Odhan IK et al. Rhodiola rosea L. and Alzheimer’s disease: from farm to pharmacy. Phytother Res 2016; 30: 532-539 DOI: 10.1002/ptr.5569.
- Ma GP, Zhang Q, Xu MB et al. Rhodiola rosea L. improves Learningand memory functions: preclinical evidence and possible mechanisms. Front Pharmacol (eCollection) 2018; 9. DOI: 10.3389/fphar.2018.01415.
- Bystritsky A, Kerwin BA, Feusner JD. A pilot study of Rhodiola rosea (Rhodax®) for generalized anxiety disorder (GAD). J Alter Complement Med 2008; 14: 175-180. DOI: 10.1089/acm.2007.7117.
- Phan CW, David P, Sabaratnam V. Edible and medicinal mushrooms: emerging brain food for the mitigation of neurodegenerative diseases. J Med Food 2017; 20: 1-10. DOI: 10.1089/jmf.2016.3740.
- Li IC, Lee LY, Tzeng TT, et al. Neurohealth properties of Hericium erinaceus mycelia enriched with erinacines. Behav Neurol 2018:5802634. DOI: 10.1155/2018/5802634.
- Chong PS, Fung ML, Wong KH, Lee WL. Therapeutic potential of Hericium erinaceus for depressive disorder. I J Mol Sci 2019; 25: 163. DOI: 10.3390/ijms21010163.
- Nagano M, Shimizu K, Kondo R, et al. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed Res 2010; 31: 231-237. DOI: 10.2220/biomedres.31.231.
- Zhang J, An S, Hu W, et al. The neuroprotective properties of Hericium erinaceus in glutamate-damaged differential PC12 cells and in Alzheimer’s disease mouse model. Int J Mol Sci 2016; 17: 1810. DOI: 10.3390/ijms17111810.
- Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease – but how and why? Prostaglandins Leukot Essent Fatty Acids 2008; 78: 11-19. DOI: 10.1016/j.plefa.2007.10.006.
- Engelmann B, Wiedmann MKH. Cellular phospholipid uptake: flexible paths to coregulate the functions of intracellular lipids. Biochem Biophys Acta 2010; 1801: 609-616. DOI: 10.1016/j.bbalip.2010.02.013.

